|
|
| | 5-BROMO-2-METHOXYPHENYLACETONITRILE Basic information |
| Product Name: | 5-BROMO-2-METHOXYPHENYLACETONITRILE | | Synonyms: | 5-BROMO-2-METHOXYPHENYLACETONITRILE;Benzeneacetonitrile, 5-broMo-2-Methoxy-;5-BroMo-2-Methoxybenzyl cyanide;5-Bromo-2-methoxybenzyl cyanide, 4-Bromo-2-(cyanomethyl)anisole;2-(5-broMo-2-Methoxyphenyl)acetonitrile;5-Bromo-2-methoxybenzeneacetonitrile | | CAS: | 7062-40-0 | | MF: | C9H8BrNO | | MW: | 226.07 | | EINECS: | | | Product Categories: | Aromatic Nitriles | | Mol File: | 7062-40-0.mol | 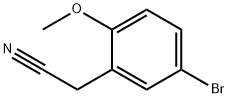 |
| | 5-BROMO-2-METHOXYPHENYLACETONITRILE Chemical Properties |
| Melting point | 61-63°C | | Boiling point | 182.5-183 °C | | density | 1.450±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | Appearance | White to off-white Solid |
| Hazard Codes | Xi | | Risk Statements | 20/21/22-36/37/38 | | Safety Statements | 26-36/37/39 | | RIDADR | 3439 | | Hazard Note | Irritant | | HazardClass | IRRITANT, IRRITANT-HARMFUL | | HS Code | 2926907090 |
| | 5-BROMO-2-METHOXYPHENYLACETONITRILE Usage And Synthesis |
| Uses | 2-(5-Bromo-2-methoxyphenyl)acetonitrile is a useful intermediate used in the synthesis of 5-bromo-2-methoxyphenylacetonitrile and some of its derivatives. | | Synthesis Reference(s) | Journal of Medicinal Chemistry, 38, p. 2050, 1995 DOI: 10.1021/jm00012a004 | | Synthesis | GENERAL METHODS: N-bromosuccinimide (NBS, 1.5 eq., 0.3 mmol), catalyst (10 mol%, 0.02 mmol) and acetonitrile (CH3CN, 1.0 mL) were sequentially added to the reaction tube, followed by o-methoxybenzeneacetonitrile (0.2 mmol). The reaction mixture was stirred at room temperature in a dark environment for 12 hours. Upon completion of the reaction, the reaction was quenched by the addition of saturated sodium thiosulfate (Na2S2O3) aqueous solution (2 mL). The reaction mixture was extracted with ethyl acetate (3.5 mL), and the combined organic phases were washed with saturated brine (10 mL), dried over anhydrous sodium sulfate (Na2SO4), and filtered through a diatomaceous earth pad. The filtrate was concentrated under reduced pressure and the residue was purified by fast chromatography on silica gel column with the eluent of petroleum ether/dichloromethane (5:1, v/v) to afford the target product 5-bromo-2-methoxyphenylacetonitrile. | | References | [1] Tetrahedron, 2017, vol. 73, # 50, p. 7105 - 7114 |
| | 5-BROMO-2-METHOXYPHENYLACETONITRILE Preparation Products And Raw materials |
|