|
ChemicalBook Optimization Suppliers |
| 名前: |
BEST-REAGENT |
| 電話番号: |
400-1166-196 18981987031 |
| 電子メール: |
cdhxsj@163.com |
|
| 化学名: | ベノミル | | 英語化学名: | Benomyl | | 别名: | tersan1991;uzgen;Benomyl? benomyl 50 w;Benosan;Benzimidazolecarbamic acid, 1-(butylcarbamoyl)-, methyl ester;Carbamic acid, 1-(butylcarbamoyl)-1H-benzimidazol-2-yl-, methyl ester;carbamic acid, methyl-, 1-(butylcarbamoyl)-2-benzimidazolyl ester;fibenzol | | CAS番号: | 17804-35-2 | | 分子式: | C14H18N4O3 | | 分子量: | 290.32 | | EINECS: | 241-775-7 | | カテゴリ情報: | Fungicide | | Mol File: | 17804-35-2.mol | 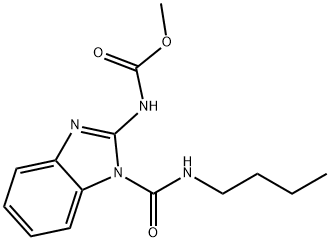 |
| 融点 | >300 °C(lit.) | | 沸点 | 432.41°C (rough estimate) | | 比重(密度) | 1.1648 (rough estimate) | | 蒸気圧 | <5.0 x 10-6 Pa (25 °C) | | 屈折率 | 1.6000 (estimate) | | 貯蔵温度 | APPROX 4°C | | 溶解性 | DMF: 30 mg/ml; DMF:PBS (pH 7.2) (1:3): 0.25 mg/ml; DMSO: 5 mg/ml; Ethanol: Slightly soluble | | 酸解離定数(Pka) | 4.2 (for carbendazim) | | 外見 | Solid | | 色 | Colorless | | 水溶解度 | <0.1 g/100 mL at 20 ºC | | Merck | 13,1042 | | BRN | 825455 | | 暴露限界値 | OSHA PEL: TWA 15 mg/m3 (total dust), 5 mg/m3 (respirable fraction);
ACGIH TLV: TWA 10 mg/m3. | | CAS データベース | 17804-35-2(CAS DataBase Reference) | | NISTの化学物質情報 | Benomyl(17804-35-2) | | EPAの化学物質情報 | Benomyl (17804-35-2) |
| 外観 | 白色~うすい黄色, 結晶性粉末~粉末 | | 溶解性 | 水3.6(pH 5)、2.9(pH 7)、1.9(pH 9)(mg/ℓ、室温)。クロロホルム94、DMF 53、アセトン18、キシレン10、エタノール4、ヘプタン0.4(g/ ㎏、25℃)(和光純薬時報 Vol.82, No.3(2014), p12)(ANALYTICAL CIRCLE 2014.9/No.74, p14)アセトンに溶け、水及びエタノールにほとんど溶けない。 | | 解説 | ベノミル.ベンレートともいう.2-メチルイソチオ尿素と2モル量のクロロギ酸メチルとを水酸化ナトリウムの存在下で反応させ,2-メチル-1,3-ビス(メトキシカルボニル)イソチオ尿素とし,ついでo-フェニレンジアミンと反応させて,N-(ベンズイミダゾール-2-イル)カルバミン酸メチル(MBC)とし,引き続きイソシアン酸ブチルを反応させると得られる.無色の結晶.分解点140 ℃.水溶解度2.9 mg L-1(pH 7,20 ℃).通常の有機溶媒に難溶.ベノミルは広範囲の植物病原菌に卓効を示す農業用殺菌剤で,植物体内でMBCに変換されて,チューブリンに結合して核分裂を阻害する.LD50 5000 mg/kg(ラット,経口). | | 用途 | 農薬(殺虫殺菌剤) | | 使用上の注意 | アルゴン封入 | | 説明 | Benomyl, a tan-coloured crystalline solid/powder, is a systemic fungicide with a characteristic
odour. It belongs to the benzimidazole family. Benomyl decomposes at high
temperature. Benomyl is essentially insoluble in water. It is stable under normal storage
conditions but will decompose to carbendazim in water. On decomposition by heat, benomyl
produces toxic fumes including nitrogen oxides.
Benomyl is a systemic and broad-spectrum fungicide that is currently registered for use
in more than 50 countries on more than 70 crops for the control of diseases in fruit trees, nut
crops, vegetables, cereals, tropical crops and ornamentals, turf, and many field crops. Benomyl
is marketed as a wettable powder and as a dry flowable formulation (dispersible granules). | | 化学的特性 | Benomyl is a white crystalline solid. Faint acrid
odor. | | 使用 | Benomyl is a systemic fungicide with both protective and curative
activities. It is effective against more than 190 different fungal diseases in
stone fruits, pome fruits, tropical and subtropical fruit crops, grapes, fruiting
vegetables, cereals, etc. It is effective against fungal diseases caused by
Ascomycetes and Basidiomycetes spp., including leaf spots, blotches and
blights; fruit spots and rots; sooty mould; scabs, bulb, corm and tuber
decays; blossom blights; powdery mildew; certain rusts; common soil
borne crown and root rots. | | 使用 | Post-harvest systemic fungicide used to control fungi and mildew on cotton, roses,
soft fruits, tomatoes, cucumbers and other vegetables. | | 使用 | Fungicide; ascaricide. | | 定義 | ChEBI: A member of the class of benzimidazoles that is the methyl ester of [1-(butylcarbamoyl)-1H-benzimidazol-2-yl]carbamic acid. A foliar fungicide used to control a wide range of Ascomycetes and Fungi Imperfecti in
a wide range of crops. | | 一般的な説明 | Colorless to white crystals or off-white powder. Faint acrid odor. | | 空気と水の反応 | Insoluble in water. | | 反応プロフィール | Benomyl is incompatible with strong acids, peroxides and strong oxidizers. Decomposed by strong alkalis. Also decomposes on storage with water . | | 危険性 | High toxicity by ingestion. Upper respira-
tory tract irritant, male reproductive, testicular, and
embryo/fetal damage. Possible carcinogen. | | 健康ハザード | Mildly toxic in rodent by ingestion, inhalation, and absorption through skin; large dosescan produce effects of carbamate poisoning;teratogenic and mutagenic effects reported;carcinogenic potential not known; mild skinirritant.
LD50 oral (rat): 10,000 mg/kg
LD50 skin (mouse): 5600 mg/kg
LD50 skin (wild bird): 100 mg/kg
Benomyl metabolizes to carbendazim(MBC) and 5-HBC. Animal studies indicatedthat benomyl and its metabolites rapidlyeliminated out within 24 hours of exposure.They do not accumulate in tissues over long�term exposure.. | | 火災危険 | Literature sources indicate that Benomyl is nonflammable. | | 农业用途 | Fungicide: Used as a pre-harvest systemic fungicide and as a
post-harvest dip. Used on arable and vegetable crops, apples,
soft fruit, nuts, mushrooms, lettuce, tomatoes and turf.
In California, the top five crops for which benomyl is used
are pistachios, table and raisin grapes, almonds, strawberries
and wine grapes. All uses of benomyl products in the
United States was phased out with a deadline of December
31, 2003. Not approved for use in EU countries. | | 製品名 | ABORTRINE®; AGROCITE®;
ARILATE®; BBC 6597®; BENEX®; BENLAT®[C];
BENLATE®, withdrawn 5/7/01; BENLATE 50®;
BENLATE 50 W®; BENLATE 50WP®; BENOMYL®
50 W; BENOSAN®; D 1991®[C]; F 1991®;
FUNDAZOL®; FUNGICIDE 1991®; FUNGACIDE
D-1991®; FUNGOCHROM®; TARSAN®; TERSAN®;
TERSAN 1991®; UZGN® | | 接触アレルゲン | Benomyl is a fungicide, derived from benzimidazole.
Cases of sensitization were reported in horticulturists and
florists. It is however, at most, a weak sensitizer, with
possible false-positive patch reactions, or with crossreactions
after previous exposure to other fungicides. | | 安全性プロファイル | Poison by ingestion.
Mildly toxic by inhalation. Experimental
teratogenic and reproductive effects. Human
mutation data reported. A human skin irritant. When heated to decomposition it
emits toxic fumes of NO,. See also
CARB AMATES. | | 職業ばく露 | Benomyl is used as an agricultural
chemical and pesticide, pharmaceutical, and veterinary drug. | | 応急処置 | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. | | 発がん性 | Benomyl was genotoxic, causing chromosome
aberrations in vitro and in vivo, but it does
not directly act with DNA. | | 環境運命予測 | Biological. Mixed cultures can grow on benomyl as the sole carbon source. It was
proposed that benomyl degraded to butylamine and methyl 2-benzimidazole-carbamate
(MBC), the latter undergoing further degradation to 2-aminobenzimidazole then to carbon
dioxide and other products (Fuchs and de Vries, 1978).
Soil. In soil and water, benomyl is transformed to methyl-2-benzimidazole and 2-
aminobenzimidazole (Rhodes and Long, 1974; Ramakrishna et al., 1979; Rajagopal et al.,
1984). Benomyl is easily hydrolyzed in soil to methyl-2-benzimidazole carbamat
Plant. On apple foliage treated with a Benlate formulation, benomyl was transformed
to MBC. Benomyl dissipated quickly and the reported half-life on foliage was 3–7 days
(Chiba and Veres, 1981).
Chemical/Physical. In aqueous solutions, especially in the presence of acids, benomyl
hydrolyzes to the strongly fungicidal methyl-2-benzimidazolecarbamate (carbendazim)
(Clemons and Sisler, 1969; Peterson and Edgington, 1969; Zbozinek, 1984; Cremlyn,
1991; Worthing and Hance, 1991) and butyl isocyanate (Zbozinek, 1984; Worthing and
Hance, 1991). The latter is unstable in water and decomposes to butylamine and carbon dioxide (Zbozinek, 1984). In highly acidic and alkaline aqueous solutions (pH <1 and pH
>11), benomyl is completely converted to 3-butyl-2,4-dioxo-[1,2-a]-s-triazinobenzimida zole (STB) with smaller quantities of methyl 2-benzimidazolecarbamate (MBC). In addi tion 1-(2-benzimidazolyl)-3-n-butylurea was identified but only under highly alkaline
conditions (Singh and Chiba, 1985).
Emits toxic fumes of nitrogen oxides when heated to decomposition (Lewis, 1990). | | 代謝経路 | When benlate is degraded by the singlet oxygen
photoreaction either in methanol or in aqueous
hydrochloric solutions, carbendazim is identified as a
major degradation product with the other nine
degradation products from both reactions, including 2-guanidinobenzimidazole, benzimidazole, and 2,4'-and
2,5'-bibenzimidazoles which are identical to the
photodegradation products of carbendazim by the
singlet oxygen photoreaction in aqueous hydrochloric
acid solution. | | 貯蔵 | Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Store in a cool, dry place or ina refrigerator away from strong bases, strong acids, heat. | | 輸送方法 | UN3077 Environmentally hazardous substances,
solid, n.o.s., Hazard class: 9; Labels: 9—Miscellaneous
hazardous material, Technical Name Required. | | Degradation | Benomyl (1) was stable under strongly acidic conditions (i.e. 5N HCl;
Singh et al., 1992). Under mildly acidic conditions, benomyl was converted
into carbendazim (MBC, 2) through the loss of n-butyl isocyanate
(3). The mechanism of this reaction involves interaction between the
electron pair at the N-1 position and the proton on the n-butylcarbamoyl
nitrogen. The formation of this hydrogen bond leads to proton abstraction
and the release of 3 (Calmon and Sayag, 1976a). Under alkaline conditions,
the formation of MBC became secondary to the formation of 3-
butyl-2,4-dioxo-s-triazino[1,2-a]benzimidazole(4 ) such that compound 4
was the major degradate at pH 13 (Calmon and Sayag, 1976b; Singh and
Chiba, 1985). The DT50 values of benomyl at 25 °C in pH 5,7 and 9 solutions
were 3.5, 1.5 and <1 hour, respectively (WHO, 1993). The relative
stability of benomyl in strongly acidic solution was attributed to the
protonation of the molecule (Singh et al., 1990).
Carbendazim (2) decomposed under alkaline conditions via the cleavage
of the amide linkage to yield 2-aminobenzimidazole (2-AB, 5)
(Watkins, 1976; Zbozinek, 1984). In pH 9 solution, the hydrolytic DT50 for
MBC was ca. 54 days (WHO, 1993). Under extreme alkaline conditions,
compound 4 was converted into 1-(2-benzimidazolyl)-3-n-butylurea (6)
via the cleavage of the carbamoyl moiety (White et al., 1973). Butyl
isocyanate (3), once formed, hydrolysed rapidly in aqueous solution to
yield n-butylcarbamic acid (7) and n-butylamine (8) (Calmon and Sayag,
1976a). These products are shown in Scheme 1.
Photolysis was not a significant degradation pathway for benomyl in
sterile pH 5 solution (Powley, 1985) under natural sunlight. | | 不和合性 | Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides (forms toxic oxides of nitrogen).
Decomposed in water or otherwise moist conditions. | | 廃棄物の処理 | In accordance with
40CFR165, follow recommendations for the disposal of
pesticides and pesticide containers. Must be disposed properly
by following package label directions or by contacting
your local or federal environmental control agency, or by
contacting your regional EPA office. |
|