|
ChemicalBook Optimization Suppliers |
|
| 化学名: | ポマリドマイド | | 英語化学名: | Pomalidomide | | 别名: | 4-Amino-2-(2,6-dioxo-3-piperidyl)isoindoline-1,3-dione;Pomalidomide;Pomalidomide(CC-4047);ActiMid;1H-Isoindole-1,3(2H)-dione,4-aMino-2-(2,6-dioxo-3-piperidinyl)-;Pomalidomide(CC-4047,Actimid);1,3-dioxo-2-(2,6-dioxopiperidin-3-yl)-4-aminoisoindoline;3-amino-N-(2,6-dioxo-3-piperidyl)phthalamide | | CAS番号: | 19171-19-8 | | 分子式: | C13H11N3O4 | | 分子量: | 273.24 | | EINECS: | 805-902-5 | | カテゴリ情報: | Inhibitor;Inhibitors;CC-4047 | | Mol File: | 19171-19-8.mol | 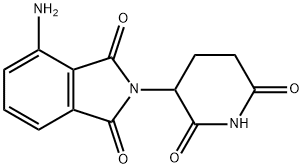 |
| 融点 | 318.5 - 320.5° | | 沸点 | 582.9±45.0 °C(Predicted) | | 比重(密度) | 1.570±0.06 g/cm3(Predicted) | | 貯蔵温度 | 2-8°C | | 溶解性 | DMSO: ≥14mg/mL | | 酸解離定数(Pka) | 10.75±0.40(Predicted) | | 外見 | powder | | 色 | yellow | | Merck | 14,135 | | 安定性: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. | | InChI | InChI=1S/C13H11N3O4/c14-7-3-1-2-6-10(7)13(20)16(12(6)19)8-4-5-9(17)15-11(8)18/h1-3,8H,4-5,14H2,(H,15,17,18) | | InChIKey | UVSMNLNDYGZFPF-UHFFFAOYSA-N | | SMILES | C1(=O)C2=C(C(N)=CC=C2)C(=O)N1C1CCC(=O)NC1=O |
| | ポマリドマイド Usage And Synthesis |
| 外観 | 白色~黄色~緑色粉末~結晶 | | 用途 | ポマリドミド(Pomalidomide)は、サリドマイドの誘導体であり、血管新生阻害作用と免疫調節作用を有する医薬品である。ボルテゾミブ?レナリドミド治療抵抗性または治療後に再発した多発性骨髄腫の治療に用いられる。
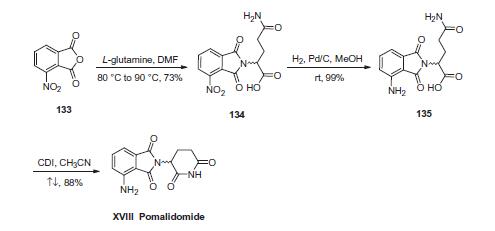
ポマリドミドは直接的に血管新生を阻害すると共に骨髄腫細胞の成長を阻害する。この二重効果が作用の本質であり、ロリプラムやペントキシフィリン(英語版)などのTNF-α阻害薬が骨髄腫細胞の阻害も血管新生の阻害もしないことに比べて効果的である。 | | 効能 | 抗悪性腫瘍薬, 免疫調節薬 | | 商品名 | ポマリスト (セルジーン) | | 説明 | In February 2013, the US FDA approved pomalidomide (also known as CC4047) for the treatment of multiple myeloma (MM) in patients with disease progression after receiving other cancer therapeutics. Pomalidomide is a 4-amino analog of thalidomide with enhanced potency and an improved toxicity profile. Pomalidomide and thalidomide exert their effects by modulation of immunity, inhibition of angiogenesis, interference with the bone/tumor microenvironment, and inhibition of the cereblon protein. Pomalidomide potently inhibited in vitro proliferation in a variety of human MM cell lines, IC50~10 nM, while thalidomide showed almost no inhibition up to 100 μM. In mouse MM tumor models, 50 mg/kg daily doses of pomalidomide resulted in marked inhibition of tumor growth after 15 days of treatment and complete regression in 3–6 weeks versus thalidomide-treated controls at the same dose. Pomalidomide is prepared by condensation of 4-nitrophthalic anhydride with 3-aminopiperidine-2,6-dione followed by catalytic hydrogenation of the nitro group. | | 化学的特性 | Yellow Solid | | Originator | Celgene Corporation (United States) | | 使用 | Pomalidomide is a second generation immunomodulator, TNF-α inhibitor, and thalidomide analog. An inhibitor of LPS-induced TNFαrelease. | | 使用 | Pomalidomide is a thalidomide derivative, a potent inhibitor of TNF-α production. It is an antiinflammatory and antitumor agent used in the treatment of multiple myeloma. | | 使用 | Pomalidomide inhibits LPS-induced TNF-α release with IC50 of 13 nM | | 定義 | ChEBI: An aromatic amine that is thalidomide substituted at position 4 on the isoindole ring system by an amino group. Used for the treatment of multiple myeloma in patients who failed to respond to previous therapies. | | brand name | Pomalyst | | Biochem/physiol Actions | Pomalidomide is an effective fetal hemoglobin (HbF) inducer that downregulates the key γ-globin repressors, SRY-box transcription factor 6 (SOX6), and BAF chromatin remodeling complex subunit (BCL11A). | | 臨床応用 | Treatment of multiple myeloma | | 合成 | First, condensation of commercially available 3-nitrophthalic
anhydride (133) and L-glutamine in warm DMF gave nitrophthalimide
134. Although the authors from Celgene do not
explicitly describe the racemization of the stereocenter derived
from L-glutamine, scrambling of the stereocenter has been
reported during this step under neutral conditions at elevated
temperatures. Next, hydrogenative reduction of the nitro group
furnished the anilinophthalimide 135, and this was followed by
treatment with CDI in refluxing acetonitrile to secure the piperidone
dione and ultimately furnish pomalidomide (XVIII) as the
racemate in 87% overall yield from 134.
| | target | TNF-α | | 薬物相互作用 | Potentially hazardous interactions with other drugs
Antidepressants: concentration increased by
fluvoxamine. | | 代謝 | Mainly metabolised in the liver by the cytochrome P450
isoenzymes CYP1A2 and CYP3A4, with CYP2C19 and
CYP2D6 playing a minor role.
Following a single oral administration of
[14C]-pomalidomide (2 mg) to healthy subjects,
approximately 73% and 15% of the radioactive dose
was eliminated in urine and faeces, respectively, with
approximately 2% and 8% of the dosed radiocarbon
eliminated as pomalidomide in urine and faeces. | | 貯蔵 | Store at +4°C | | 参考文献 | 1) Lopez-Girona?et al.?(2012),?Cereblon is direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide; Leukemia,?26?2326
2) Zhu?et al.?(2013),?Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma; Leukemia Lymphoma,?54?683
3) Donovan?et al.?(2018),?Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane Radial Ray syndrome; Elife,?7?e38430
4) Winter?et al.?(2015),?DRUG DEVELOPMENT. Phthalimide conjunction as a strategy for in vivo target protein degradation; Science,?348?1376
5) Lohbeck and Miller (2016),?Practical synthesis of a phthalimide-based Cereblon ligand to enable PROTAC development; Bioorg. Med. Chem. Lett.,?26?5260 |
|