|
ChemicalBook Optimization Suppliers |
|
| 化学名: | イバカフトール | | 英語化学名: | VX-770 | | 别名: | Ivacaftor, >=98%;3-QuinolinecarboxaMide, N-[2,4-bis(1,1-diMethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxo-;N-[2,4-Bis(tert-butyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxo-3-quinolinecarboxamide;VX 770, Ivacaftor;Ivacaftor-D4;VX-770(Ivacattor);IVACAFTOR (VX-770);VX 770;N-(2,4-DITERT-BUTYL-5-HYDROXYPHENYL)-4-OXO-1H-QUINOLINE-3-CARBOXAMIDE | | CAS番号: | 873054-44-5 | | 分子式: | C24H28N2O3 | | 分子量: | 392.49 | | EINECS: | | | カテゴリ情報: | API;Inhibitors | | Mol File: | 873054-44-5.mol | 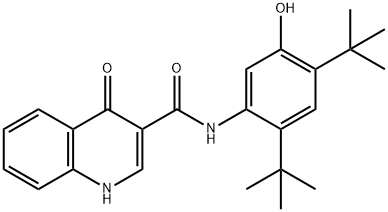 |
| 融点 | 212-215°C | | 沸点 | 550.4±50.0 °C(Predicted) | | 比重(密度) | 1.187 | | 貯蔵温度 | Refrigerator | | 溶解性 | DMSO (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) | | 酸解離定数(Pka) | 11.08±0.23(Predicted) | | 外見 | Solid | | 色 | White to Light Brown | | InChI | InChI=1S/C24H28N2O3/c1-23(2,3)16-11-17(24(4,5)6)20(27)12-19(16)26-22(29)15-13-25-18-10-8-7-9-14(18)21(15)28/h7-13,27H,1-6H3,(H,25,28)(H,26,29) | | InChIKey | PURKAOJPTOLRMP-UHFFFAOYSA-N | | SMILES | N1C2=C(C=CC=C2)C(=O)C(C(NC2=CC(O)=C(C(C)(C)C)C=C2C(C)(C)C)=O)=C1 |
| | イバカフトール Usage And Synthesis |
| 効能 | CFTR増強薬 | | 説明 | In January 2012, the US FDA approved ivacaftor for the treatment of cystic
fibrosis (CF) in patients who have the G551D mutation of the CF transmembrane regulator (CFTR) and are at least 6 years old. Ivacaftor (also known as VX-770) is a CFTR potentiator that increases the open probability of CFTR, thus increasing chloride
secretion particularly in the 5% of CF patients with the G551D/F508 gating/
processing mutation. Ivacaftor was discovered by medicinal chemistry optimization of a lead scaffold identified through high-throughput screening of a 228,000 compound collection. In cultured bronchial epithelial cells from a CF patient with F508del, ivacaftor increased chloride secretion
(EC50=81 nM). Preparation of ivacaftor is accomplished via a multistep
synthesis oftwointermediates, 4-oxo-1,4-dihydroquinoline-3-carboxylic acid
and 5-amino-di-tert-butylphenyl methyl carbonate, which are coupled using
propane phosphonic acid anhydride (T3P) to afford the amide; deprotection of
the phenol then provides ivacaftor. | | Originator | Vertex Pharmaceuticals (United States) | | 使用 | Ivacaftor (VX-770, Kalydeco) is a potentiator of CFTR targeting G551D-CFTR and F508del-CFTR with EC50 of 100 nM and 25 nM, respectively | | 使用 | Ivacaftor is used in the treatment of cystic fibrosis. | | 定義 | ChEBI: An aromatic amide obtained by formal condensation of the carboxy group of 4-oxo-1,4-dihydroquinoline-3-carboxylic acid with the amino group of 5-amino-2,4-di-tert-butylphenol. Used for the treatment of cystic fibrosis. | | brand name | Kalydeco | | 臨床応用 | Vertex’s ivacaftor was granted breakthrough therapy designation by the FDA in January 2012 for
cystic fibrosis (CF) patients who bear the G551D mutation in the Cycstic Fibrosis Transmembrane
Regulator (CFTR) gene. This CFTR mutation occurs in roughly 4% of the 30,000 people living with
CF in the United States. While the compound has been identified as a potentiator in cell-based assays,
its mechanism of action is as yet unknown. | | 合成 | Several patents describe a synthesis of
ivacaftor, only one demonstrates the synthesis on scale and includes yields, which is depicted in the
scheme. Beginning with treatment of commercial di-tert-butylphenol derivative 91 with ethyl chloroformate,
the synthesis of carbonate 92 was achieved in quantitative yield. Nitration of 92 provided the desired nitroarene regioisomer 93 in 57% yield which was isolated by recrystallization. Reduction of the newlyinstalled
nitro group and subsequent amide bond formation via reaction with commercially available
acid chloride 94 produced amide 95 in 53% yield over the two step sequence. Finally, cleavage of the
carbonate unmasked the phenol to furnish ivacaftor (XV) in 96% yield. 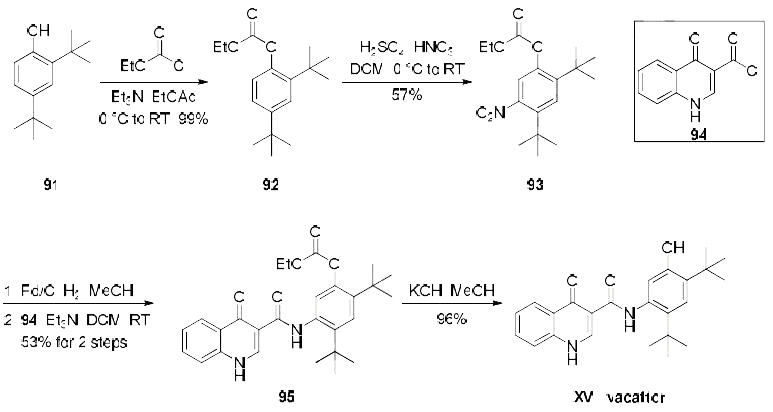
| | target | G551D-CFTR | | 参考文献 | 1. van goor f1, hadida s, grootenhuis pd, burton b, cao d, neuberger t, turnbull a, singh a, joubran j, hazlewood a, zhou j, mccartney j,arumugam v, decker c, yang j, young c, olson er, wine jj, frizzell ra, ashlock m, negulescu p. rescue of cf airway epithelial cell function in vitro by a cftr potentiator, vx-770. proc natl acad sci u s a. 2009 nov 3;106(44):18825-30. 2. vachel l1, norez c, becq f, vandebrouck c. effect of vx-770 (ivacaftor) and oag on ca2+ influx and cftr activity in g551d and f508del-cftr expressing cells. j cyst fibros. 2013 dec;12(6):584-91 |
|