|
|
| | N-(3,5-DiMethyladaMantan-1-yl)forMaMide Basic information |
| Product Name: | N-(3,5-DiMethyladaMantan-1-yl)forMaMide | | Synonyms: | 1-ForMylaMino-3,5-diMethyladaMantane;N-(3,5-DiMethyladaMantan-1-yl)forMaMide;N-(3,5-DiMethyltricyclo[3.3.1.13,7]dec-1-yl)forMaMide;Memantine Related Compound E (15 mg) (N-3,5-Dimethyladamantan-1-yl formamide);N-(3,5-dimethyl-1-adamantyl)formamide;N-(3,5-DiMethyladaMantan-1-yl);FORMAMIDE, N-(3,5-DIMETHYLTRICYCLO[3.3.1.13,7]DEC-1-YL)-;Memantine USP Related Compound E | | CAS: | 351329-88-9 | | MF: | C13H21NO | | MW: | 207.31 | | EINECS: | 609-072-3 | | Product Categories: | Amines;Impurities;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 351329-88-9.mol | 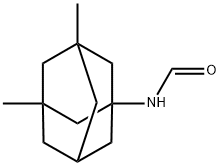 |
| | N-(3,5-DiMethyladaMantan-1-yl)forMaMide Chemical Properties |
| Melting point | 69.5℃ | | Boiling point | 325.6±9.0 °C(Predicted) | | density | 1.07±0.1 g/cm3(Predicted) | | storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Solid | | pka | 16.08±0.60(Predicted) | | color | White to Off-White | | InChI | InChI=1S/C13H21NO/c1-11-3-10-4-12(2,6-11)8-13(5-10,7-11)14-9-15/h9-10H,3-8H2,1-2H3,(H,14,15) | | InChIKey | NYQWYYMEIBHRSB-UHFFFAOYSA-N | | SMILES | C(NC12CC3(C)CC(CC(C)(C3)C1)C2)=O |
| | N-(3,5-DiMethyladaMantan-1-yl)forMaMide Usage And Synthesis |
| Chemical Properties | White Solid | | Uses | N-Formyl Memantine is an impurity of Memantine (M218000). Memantine impurity E per USP. | | Synthesis | GENERAL METHOD: To a 17 mL stainless steel pressure microreactor or glass vial under argon protection, 0.3 mmol of manganese-containing catalyst, 10 mmol of 1-bromo-3,5-dimethyladamantane (I), and 30 mmol of formamide were sequentially added. The reaction mixture was heated with stirring at 120-130°C for 3-4 hours. After completion of the reaction, the reactor was cooled to room temperature and opened. The reaction mixture was washed with water and the reaction product was subsequently extracted with 33 mL of dichloromethane. The solvent was removed by distillation under reduced pressure and the residue was purified by recrystallization. Further purification by silica gel column chromatography (eluent: hexane-ethyl acetate mixed solvent) afforded N-(3,5-dimethyladamantan-1-yl)formamide (II). The yield of the product was 78% and the melting point was 69-70°C (hexane recrystallization). Its physicochemical properties and NMR spectral data were in agreement with those reported in the literature [7]. | | References | [1] Russian Journal of General Chemistry, 2015, vol. 85, # 7, p. 1771 - 1772
[2] Zh. Obshch. Khim., 2015, vol. 85, # 7, p. 1210 - 1211,2
[3] Patent: WO2010/15415, 2010, A1. Location in patent: Page/Page column 16-17
[4] Patent: WO2010/83996, 2010, A1. Location in patent: Page/Page column 20-21 |
| | N-(3,5-DiMethyladaMantan-1-yl)forMaMide Preparation Products And Raw materials |
|