|
ChemicalBook Optimization Suppliers |
|
| 化学名: | | | 英語化学名: | Vonoprazan fumarate | | 别名: | 1-[5-(2-fluorophenyl)-1-pyridin-3-ylsulfonylpyrrol-3-yl]-N-methylmethanamine;AK-438;vonoprazan(tak-438);fuMarate vonoprazan;1H-Pyrrole-3-methanamine, 5-(2-fluorophenyl)-N-methyl-1-(3-pyridinylsulfonyl)-, 2-butenedioate (1:1);5-(2-Fluorophenyl)-N-methyl-1-(3-pyridinylsulfonyl)-1H-pyrrole-3-methanamine 2-butenedioate TAK 438;vonaprazan(TK-438);TAK-438
Vonoprazan fumarate | | CAS番号: | 1260141-27-2 | | 分子式: | C21H20FN3O6S | | 分子量: | 461.4634032 | | EINECS: | 250-635-4 | | カテゴリ情報: | Vonoprazan;Inhibitors;API;1260141-27-2 | | Mol File: | 1260141-27-2.mol | 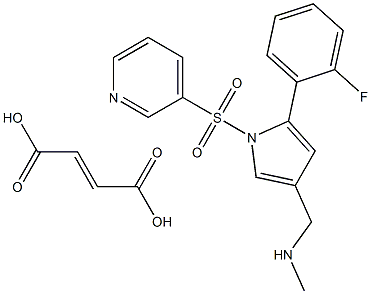 |
| 貯蔵温度 | Store at -20°C | | 溶解性 | insoluble in H2O; insoluble in EtOH; ≥18.9 mg/mL in DMSO | | 外見 | solid | | InChIKey | ROGSHYHKHPCCJW-WLHGVMLRSA-N | | SMILES | N1(S(C2=CC=CN=C2)(=O)=O)C(C2=CC=CC=C2F)=CC(CNC)=C1.C(O)(=O)/C=C/C(O)=O |
| | Vonoprazan fumarate Usage And Synthesis |
| 説明 | Vonoprazan fumarate (Takecab®), discovered and developed by
Takeda and Otsuka, was approved by the PMDA of Japan in
December 2014, and is indicated for the treatment of gastric ulcer,
duodenal ulcer and reflux esophagitis. Vonoprazan fumarate has
a novel mechanism of action called potassium-competitive acid
blockers, which competitively inhibit the binding of potassium
ions to H+, K+-ATPase (also known as the proton pump) in the final
step of gastric acid secretion in gastric parietal cells. Vonoprazan
does not inhibit Na+, K+-ATPase activity even at concentrations 500
times higher than that of their IC50 values against gastric H+,
K+-ATPase activity. Furthermore, the drug is unaffected by the
gastric secretory state, unlike PPIs. | | 説明 | Vonoprazan is a selective, reversible, and potassium-competitive proton pump inhibitor that inhibits gastric H+/K+ ATPase (IC50 = 17 nM) but does not inhibit porcine Na+/K+ ATPase activity when used at a concentration of 10 μM. It maintains its inhibitory effect in both weakly acidic (pH 6.5) and neutral (pH 7.5) conditions with IC50 values of 19 and 28 nM, respectively. In vivo, vonoprazan (1, 2, and 4 mg/kg) inhibits histamine-stimulated acid secretion in a dose-dependent manner in rats, with complete inhibition when administered at a dose of 4 mg/kg. It also inhibits acid secretion for more than 48 hours in dogs when administered at doses ranging from 0.1 to 1 mg/kg. | | 使用 | Vonoprazan Fumarate is a novel potassium-?competitive acid blocker for the treatment of acid-?related diseases. | | 主な応用 | Vonoprazan fumarate is an oral, newly synthesised potassium-competitive acid blocker (P-CAB) with antisecretory activity. It is also a proton pump inhibitor (PPI) reversibly inhibiting H+/K+, ATPase. It is mainly used in the treatment of acid-related diseases such as GERD and peptic ulcer disease. | | 合成 | Commercially
available 2-fluoroacetophenone (283) was brominated to yield
a-bromo-acetophenone derivative 284. This compound was
treated with ethyl 2-cyanoacetate (285) under basic conditions
to provide ketoester 286 in essentially quantitative yield. Next,
intramolecular condensation of 286 upon treatment of 4 M HCl
furnished the tri-substituted pyrrole 287 in 53% yield. Reduction
of the chloride under hydrogenolytic conditions facilitated arrival
at pyrrole 288, albeit in just 18% yield. Subsequent diisobutylaluminium
hydride (DIBAL) reduction, followed by the oxidation with
tetrapropylammonium perruthenate (TPAP) and 4-methylmorpholine
N-oxide (NMMO) afforded the corresponding aldehyde 290 in 60% yield across the 2 steps. Next, N-pyrrole substitution with pyridine-
3-sulfonyl chloride 291 gave rise to N-sulfonylpyrrole 292 in
82% yield. Reductive amination of 292 afforded amine 293, which
was treated with fumaric acid (294) via co-crystallization to provide
vonoprazan fumarate (XXXVI) in 74% for the two steps. 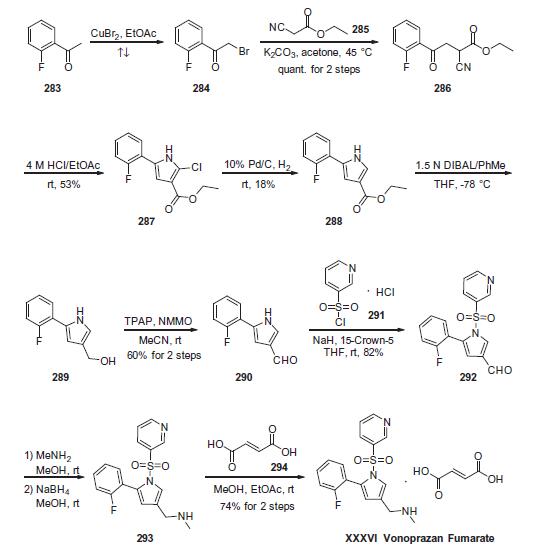
| | target | H,K-ATPase | | 参考文献 | [1]. yasunobu hori, jun matsukawa, toshiyuki takeuchi, et al. a study comparing the antisecretory effect of tak-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. journal of pharmacology and experimental therapeutics, 2011, 337:797-804.
[2]. jun matsukawa, yasunobu hori, haruyuki nishida, et al. a comparative study on the modes of action of tak-438, a novel potassium-competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. biochemical pharmacology, 2011, 81:1145-1151. |
|