|
ChemicalBook Optimization Suppliers |
|
| 主な危険性 | T | | Rフレーズ | 25-36/37/38-23/24/25 | | Sフレーズ | 26-45-36/37/39 | | RIDADR | 2811 | | WGK Germany | 3 | | RTECS 番号 | UE7570000 | | TSCA | Yes | | 国連危険物分類 | 6.1(b) | | 容器等級 | III | | HSコード | 29183000 | | 毒性 | LD50 orally in rats: 101 mg/kg (Ueno) |
| | ケトプロフェン Usage And Synthesis |
| 外観 | 白色〜ほとんど白色, 粉末 | | 溶解性 | メタノールに極めて溶けやすく、エタノール(95)又はアセトンに溶けやすく、水にほとんど溶けない。 | | 用途 | ケトプロフェン(ketoprofen)とは、抗炎症作用、鎮痛作用を有する、プロピオン酸系の酸性非ステロイド性抗炎症薬の1種で、(RS)-2-(3-ベンゾイルフェニル)プロパン酸のことである。分子内に1つキラル中心を持っているものの、医薬品として使用する際、この鏡像異性体は区別されず、ラセミ体が用いられている。
ケトプロフェンはウマや他のウマ科動物で一般的に使用される非ステロイド性抗炎症薬、解熱薬、鎮痛薬としても知られる。この他、ケトプロフェンは小動物での外科手術における鎮痛薬としても使用される。ケトプロフェンは他の非ステロイド性抗炎症薬と同様に、シクロオキシゲナーゼを阻害することによって、生体でのプロスタグランジン類の産生を抑制する。主作用は、プロスタグランジン類の中でも、特にプロスタグランジンE2の産生を抑制することによる。これによって、痛みの閾値が下がらないように(痛みを感じやすくならないように)し、また毛細血管が拡張して炎症を助長することの無いようにしている。 | | 用途 | プロピオン酸系化合物です。
シクロオキシゲナーゼ(COX)を阻害し、プ
ロスタグランジン生合成抑制作用を示します。 | | 効能 | 鎮痛薬, 抗炎症薬, 解熱薬, シクロオキシゲナーゼ阻害薬 | | 商品名 | カピステン (キッセイ薬品工業); セクター (久光製薬); セクター (久光製薬); セクター (久光製薬); ミルタックス (ニプロファーマ); モーラス (久光製薬); モーラス (久光製薬); モーラス (久光製薬); モーラス (久光製薬); モーラス (久光製薬); モーラス (久光製薬); モーラス (久光製薬); モーラス (久光製薬); モーラス (久光製薬); モーラス (久光製薬); モーラス (久光製薬) | | 確認試験 | (1) 本品のメタノール溶液(1→200000)につき,紫外可視
吸光度測定法〈2.24〉により吸収スペクトルを測定し,本品
のスペクトルと本品の参照スペクトルを比較するとき,両者
のスペクトルは同一波長のところに同様の強度の吸収を認め
る.
(2) 本品を乾燥し,赤外吸収スペクトル測定法〈2.25〉の
臭化カリウム錠剤法により試験を行い,本品のスペクトルと
本品の参照スペクトルを比較するとき,両者のスペクトルは
同一波数のところに同様の強度の吸収を認める. | | 定量法 | 本品を乾燥し,その約0.3gを精密に量り,エタノール
(95)25mLに溶かし,水25mLを加え,0.1mol/L水酸化ナト
リウム液で滴定〈2.50〉する(電位差滴定法).同様の方法で空
試験を行い,補正する. | | 純度試験 | (1) 溶状 本品1.0gをアセトン10mLに溶かすとき,液は
澄明で,液の色は次の比較液より濃くない.
比較液:塩化コバルト(Ⅱ)の色の比較原液0.6mL及び塩化
鉄(Ⅲ)の色の比較原液2.4mLの混液に薄めた希塩酸(1→
10)を加えて10mLとした液5.0mLをとり,薄めた希塩
酸(1→10)を加えて100mLとする.
(2) 重金属〈1.07〉 本品2.0gをとり,第2法により操作し,
試験を行う.比較液には鉛標準液2.0mLを加える(10ppm以
下).
(3) 類縁物質 本操作はできるだけ光を避け,遮光した容
器を用いて行う.本品20mgを移動相20mLに溶かし,試料
溶液とする.この液1mLを正確に量り,移動相を加えて正
確に50mLとする.この液1mLを正確に量り,移動相を加え
て正確に10mLとし,標準溶液とする.試料溶液及び標準溶
液20μLずつを正確にとり,次の条件で液体クロマトグラフ
ィー〈2.01〉により試験を行う.それぞれの液の各々のピー
ク面積を自動積分法により測定するとき,試料溶液から得た
ケトプロフェンに対する相対保持時間約1.5及び約0.3のピー
ク面積は,標準溶液から得たケトプロフェンのピーク面積の
4.5倍及び2倍より大きくない.また,試料溶液から得たケト
プロフェン,相対保持時間約1.5及び約0.3以外のピークの面
積は,標準溶液から得たケトプロフェンのピーク面積より大
きくなく,それらの合計面積は,標準溶液から得たケトプロ
フェンのピーク面積の2倍より大きくない. | | 乾燥減量 | 0.5%以下(0.5g,減圧,60℃,24時間). | | 説明 | Ketoprofen is a chemical that comes in the form of a white crystalline powder; odorless or nearly odorless. It is very soluble in methanol, soluble in ethanol, acetone or ether, and almost insoluble in water. The melting point is about 93-96 °C. For the arylalkanoic acid compounds. Has analgesic, anti-inflammatory and antipyretic effects. The anti-inflammatory effect is stronger than that of ibuprofen, with less side effects and low toxicity. Oral and easily absorbed from the gastrointestinal tract. After 1 administration, the peak plasma concentration can be reached in about 0.5 to 2 hours. t 1/2 is 1.6 to 1.9 hours. In the blood and plasma protein binding force is extremely strong. The excretion rate from urine is 30% to 90% within 24 hours. Mainly excreted in the form of glucuronic acid conjugates. For rheumatoid arthritis, rheumatoid arthritis, osteoarthritis, ankylosing spondylitis and gout, etc. | | 説明 | Ketoprofen (3-benzoyl-α-methylphenylacetic acid) is a 2-arylpropionic acid potent non-steroidal anti-inflammatory drug. It was first synthesized by French chemist Rhone Poulenc in 1967. In 1973, it was introduced into France and the United States as an anti-inflammatory drug. It has good effects on rheumatism, rheumatoid arthritis, myelitis and gout, and its anti-inflammatory effect is stronger than that of ibuprofen. Ibuprofen. At the same dose, its anti-inflammatory and analgesic effect is 150 times that of aspirin, its antipyretic effect is 4 times that of indomethacin and 100 times that of aspirin. Because ketoprofen has high efficacy, short half-life, It has the advantages of simple metabolism and few and mild adverse reactions, and has been widely used in the treatment of various types of pain, inflammatory symptoms, colds and post-operative anti-inflammatory analgesia. | | 化学的特性 | White Crystalline Solid | | Originator | Profenid,Specia,France,1973 | | 使用 | Natural Vitamin B12. analog | | 使用 | Anti-inflammatory; analgesic | | 使用 | Ketoprofen, a propionic acid derivative, is a nonsteroidal anti-inflammatory agent (NSAIA) with analgesic and antipyretic properties. | | 定義 | ChEBI: An oxo monocarboxylic acid that consists of propionic acid substituted by a 3-benzoylphenyl group at position 2. | | 適応症 | Ketoprofen (Orudis) is indicated for use in rheumatoid
and osteoarthritis, for mild to moderate pain, and in
dysmenorrhea. The most frequently reported side effects
are GI (dyspepsia, nausea, abdominal pain, diarrhea,
constipation, and flatulence) and CNS related
(headache, excitation). Edema and increased blood urea nitrogen have also been noted in more than 3% of
patients. Ketoprofen can cause fluid retention and increases
in plasma creatinine, particularly in the elderly
and in patients taking diuretics. | | Manufacturing Process | In an initial step, the sodium derivative of ethyl (3-benzoylphenyl)
cyanoacetate is prepared as follows: (3-benzoylphenyl)acetonitrile (170 9) is
dissolved in ethyl carbonate (900 g). There is added, over a period of 2 hours,
a sodium ethoxide solution [prepared from sodium (17.7 g) and anhydrous
ethanol (400 cc)], the reaction mixture being heated at about 105° to 115°C
and ethanol being continuously distilled. A product precipitates. Toluene (500
cc) is added, and then, after distillation of 50 cc of toluene, the product is
allowed to cool. Diethyl ether (600 cc) is added and the mixture is stirred for
1 hour. The crystals which form are filtered off and washed with diethyl ether
(600 cc) to give the sodium derivative of ethyl (3-benzoylphenyl)cyanoacetate
(131 g).
Then, ethyl methyl(3-benzoylphenyl)cyanoacetate employed as an
intermediate material is prepared as follows: The sodium derivative of ethyl
(3-benzoylphenyl)cyanoacetate (131 g) is dissolved in anhydrous ethanol (2
liters). Methyl iodide (236 g) is added and the mixture is heated under reflux
for 22 hours, and then concentrated to dryness under reduced pressure (10
mm Hg). The residue is taken up in methylene chloride (900 cc) and water
(500 cc) and acidified with 4N hydrochloric acid (10 cc). The methylene
chloride solution is decanted, washed with water (400 cc) and dried over
anhydrous sodium sulfate. The methylene chloride solution is filtered through
a column containing alumina (1,500 g). Elution is effected with methylene
chloride (6 liters), and the solvent is evaporated under reduced pressure (10
mm Hg) to give ethyl methyl(3-benzoylphenyl)cyanoacetate (48 g) in the
form of an oil.
In the final production preparation, a mixture of ethyl methyl(3-
benzoylphenyl)cyanoacetate (48 g), concentrated sulfuric acid (125 cc) and
water (125 cc) is heated under reflux under nitrogen for 4 hours, and water
(180 cc) is then added. The reaction mixture is extracted with diethyl ether
(300 cc) and the ethereal solution is extracted with N sodium hydroxide (300
cc). The alkaline solution is treated with decolorizing charcoal (2 g) and then
acidified with concentrated hydrochloric acid (40 cc). An oil separates out,
which is extracted with methylene chloride (450 cc), washed with water (100
cc) and dried over anhydrous sodium sulfate. The product is concentrated to
dryness under reduced pressure (20 mm Hg) to give a brown oil (33.8 g).
This oil is dissolved in benzene (100 cc) and chromatographed through silica
(430 g). After elution with ethyl acetate, there is collected a fraction of 21
liters, which is concentrated to dryness under reduced pressure (20 mm Hg).
The crystalline residue (32.5 g) is recrystallized from acetonitrile (100 cc) and
a product (16.4 g), MP 94°C, is obtained. On recrystallization from a mixture
of benzene (60 cc) and petroleum ether (200 cc), there is finally obtained 2-
(3-benzoylphenyl)propionic acid (13.5 g), MP 94°C. | | brand name | Actron (Bayer); Orudis (Wyeth); Oruvail (Wyeth), Alrheumat (Bayer, United Kingdom), Alrheumun (Bayer Pharma Deutschland, Germany), Gabrilen (Kreussler, Germany), Orudis (Rh?one-Poulenc Rorer, Canada, Denmark; Wyeth-Ayerst, USA). | | Therapeutic Function | Antiinflammatory | | 一般的な説明 | Ketoprofen (Orudis, Rhodis) and suprofen (Profenal) areclosely related to fenoprofen in their structures, properties,and indications. Even though ketoprofen has been approvedfor OTC use (Orudis KT, Actron), its GI side effects aresimilar to indomethacin, and therefore its useshould be closely monitored, especially in patients with GIor renal problems. | | 接触アレルゲン | Ketoprofen is an anti-inflammatory drug, used both
topically and systemically. It is above all a photoaller-
gen, responsible for photoallergic or photo-worsened
contact dermatitis, with sun-induced, progressive,
severe, and durable reactions. Recurrent photosensitiv-
ity is possible for many years. Photosensitivities are
expected to thiophene-phenylketone derivatives such
as tiaprofenic acid and suprofen, to ketoprofen esters
such as piketoprofen, and to benzophenone derivatives
(see above) such as fenofibrate and benzophenone-3.
Concomitant photosensitivities without clinical rel-
evance have been observed to fenticlor, tetrachloro-
salicylanilide, triclosan, tribromsalan, and bithionol. | | Biochem/physiol Actions | It serves as an efficient drug to treat ankylosing spondylitis, rheumatoid arthritis and osteoarthritis. It also has antipyretic and analgesic effects. Ketoprofen prevents the action of prostaglandin synthetase. | | 薬物動態学 | Ketoprofen is rapidly and nearly completely absorbed on oral administration, reaching peak plasma levels within 0.5
to 2 hours. It is highly plasma protein bound (99%) despite a lower acidity (pKa = 5.9) than some other NSAIDs. Wide
variation in plasma half-lives has been reported. It is metabolized by glucuronidation of the carboxylic acid, CYP3A4
and CYP2C9 hydroxylation of the benzoyl ring, and reduction of the keto function. | | 薬理学 | The pharmacologically
active isomer is mainly the S(+)-
enantiomer, which is available in some countries
as the trometamol (2-amino-2-(hydroxymethyl)-
1,3-propanediol) salt. As compared to
the racemate, absorption of the S(+)-isomer is
said to be faster, leading to an earlier onset of
action . The peak plasma concentration after oral administration
occurs within 2 h. Ketoprofen is
bound to plasma protein up to 99% and shows
a plasma elimination half-life of 1.5to 4 h. | | 臨床応用 | Ketoprofen, unlike many NSAIDs, inhibits the synthesis of leukotrienes and leukocyte
migration into inflamed joints in addition to inhibiting the biosynthesis of prostaglandins. It stabilizes the lysosomal
membrane during inflammation, resulting in decreased tissue destruction. Antibradykinin activity also has been
observed. Bradykinin is released during inflammation and can activate peripheral pain receptors. In addition to
anti-inflammatory activity, ketoprofen also possesses antipyretic and analgetic properties. Although it is less potent
than indomethacin as an anti-inflammatory agent and an analgetic, its ability to produce gastric lesions is about the
same. | | 安全性プロファイル | Poison by ingestion,subcutaneous, intravenous, rectal, and intraperitoneal routes. Human systemic effects by an unspecified route:headache, nausea or vomiting, and degenerative changesin the brain, changes in kidney tubules. An experimentalteratogen. | | 合成 | Ketoprofen, 2-(3-benzoyl)propionic acid (3.2.37), is synthesized from
3-methylbenzophenone, which undergoes bromination and forms 3-bromo-methylben�zophenone (3.2.33). The reduction of the resulting product by sodium cyanide gives
3-cyanomethylbenzophenone (3.2.34), which is reacted with the diethyl ester of carbonic
acid in the presence of sodium ethoxide. The resulting cyanoacetic ester derivative (3.2.25)
is alkylated by methyl iodide and the resulting product (3.2.36) undergoes acidic hydroly�sis, forming ketoprofen (3.2.37) [104¨C106]. 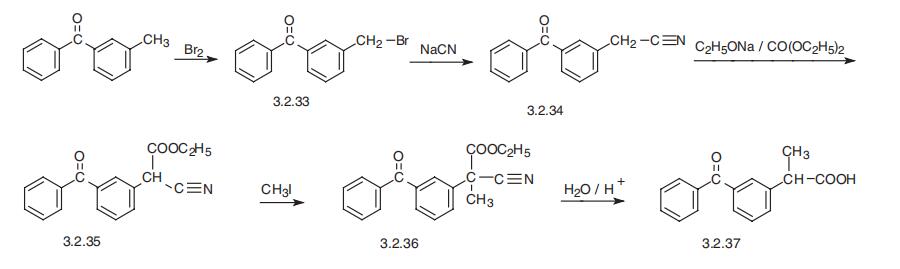
| | Veterinary Drugs and Treatments | Ketoprofen is labeled for use in horses for the alleviation of inflammation
and pain associated with musculoskeletal disorders. Like
flunixin (and other NSAIDs), ketoprofen potentially
has many other
uses in a variety of species and conditions. There are approved
dosage forms for dogs and cats in Europe and Canada. Some consider
ketoprofen to be the NSAID of choice for use short-term for
analgesia in cats. | | 薬物相互作用 | Concomitant use of alcohol or other NSAIDs after taking ketoprofen can increase gastrointestinal side effects and may cause ulcers. When ketoprofen is used together with aspirin or other salicylic acid drugs, the efficacy cannot be increased, but the incidence of gastrointestinal side effects and bleeding tendency increases. Concomitant use of ketoprofen with anticoagulants increases the risk of bleeding. Ketoprofen can enhance the effect of antidiabetic drugs and reduce the antihypertensive effect of antihypertensive drugs; ketoprofen and corticosteroids can be used together, which can significantly reduce the symptoms of inflammation. Ketoprofen should not be used with methotrexate to prevent poisoning. When ketoprofen is used with probenecid, verapamil, and nifedipine, the dose should be reduced; when ketoprofen is used with digoxin, the dose of digoxin should be adjusted. |
|