| 化学名: | ケタミン | | 英語化学名: | KETAMINE HYDROCHLORIDE SELECTIVE NMDA AN TAGO | | 别名: | 2-(2-chlorophenyl)-2-methylaminocyclohexanone;Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-;(±)-2-(o-Chlorophenyl)-2-(methylamino)cyclohexanone;Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)- (9CI);dl-Ketamine;Ketoject;NSC 70151;C07525 | | CAS番号: | 6740-88-1 | | 分子式: | C13H16ClNO | | 分子量: | 237.73 | | EINECS: | 229-804-1 | | カテゴリ情報: | | | Mol File: | 6740-88-1.mol | 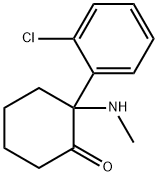 |
| 効能 | 静脈麻酔薬, NMDA受容体拮抗薬 | | Originator | Ketanest,Parke Davis,W. Germany,1969 | | 使用 | Anesthetic. | | 定義 | ChEBI: Ketamine is a member of the class of cyclohexanones in which one of the hydrogens at position 2 is substituted by a 2-chlorophenyl group, while the other is substituted by a methylamino group. It has a role as an intravenous anaesthetic, a NMDA receptor antagonist, an analgesic, a neurotoxin, an environmental contaminant and a xenobiotic. It is a member of cyclohexanones, a secondary amino compound and a member of monochlorobenzenes. | | Manufacturing Process | The 1-hydroxycyclopentyl-(o-chlorophenyl)-ketone N-methylimine used as an
intermediate is prepared as follows. To the Grignard reagent prepared from
119.0 g of cyclopentyl bromide and 19.4 g of magnesium is added 55.2 g of
o-chlorobenzonitrile. The reaction mixture is stirred for 3 days and thereafter
hydrolyzed in the usual manner. From the hydrolysis there is obtained o�chlorophenylcyclopentylketone, BP 96° to 97°C (0.3 mm), nD251.5452. To
21.0 g of the ketone is added 10.0 g of bromine in 80 ml of carbon
tetrachloride.
1-Bromocyclopentyl-(o-chlorophenyl)-ketone, BP 111° to 114°C (0.1 mm) is
isolated in the usual manner. Since it is unstable, it must be used
immediately. The bromoketone (29.0 g) is dissolved in 50 ml of liquid
methylamine. After one hour, the excess liquid methylamine is allowed to
evaporate. The organic residue is dissolved in pentane, and upon evaporation
of the solvent, 1-hydroxycyclopentyl-(o-chlorophenyl)-ketone N-methylimine,
MP 62°C, is isolated.
1-Hydroxycyclopentyl-(o-chlorophenyl)-ketone N-methylimine (2.0 g) is
dissolved in 15 ml of Decalin and refluxed for 2,5 hours. After evaporation of
the Decalin under reduced pressure, the residue is extracted with dilute
hydrochloric acid, the solution treated with decolorizing charcoal, and the
resulting acidic solution is made basic. The liberated product, 2-methylamino-
2-(o-chlorophenyl)-cyclohexanone, after crystallization from pentane-ether,
has MP 92° to 93°C. The hydrochloride of this compound has MP 262° to
263°C. | | brand name | Ketalar (Parkdale). | | Therapeutic Function | Anesthetic | | 作用機序 | The mode of action of ketamine differs from
that of the barbiturates. It suppresses the activities
of the cerebral cortex (consciousness) and
the thalamic pain pathways (analgesia). Parts
of the upper brainstem and the limbic system
are not affected (so-called dissociative analgesia).
The patient exhibits a characteristic superficial
sleep with complete elimination of pain.
Because of the psychomotor side effects, combination
with neuroleptics and tranquilizers is
necessary. Ketamine is used especially in pediatrics.
The usual preparations are 0.1 % and
0.5 % solutions. | | 臨床応用 | Ketamine is a short-acting anesthetic effective
for 5 – 30 min, depending on the amount injected.
It is suitable for diagnostic purposes and
for surgical procedures that do not require muscle
relaxation. The occasional hallucinations
that occur during anesthesia suggest a chemical
relationship to phencyclidine, which was used
as a short-acting anesthetic until identified as a
dangerous drug of abuse. | | 合成 | It is prepared by bromination of o-chlorophenyl
cyclopentyl ketone, which is then reacted
with methylamine to give the methylimino alcohol.
Thermolysis of the imino hydrochloride
yields ketamine by ring expansion :
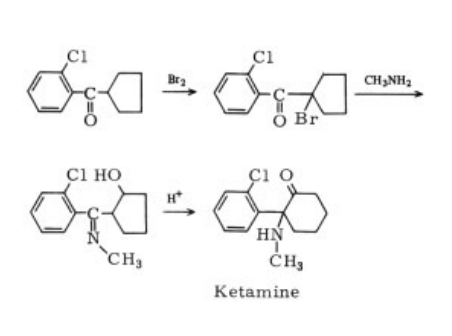 |
|