|
ChemicalBook Optimization Suppliers |
| 名前: |
Alfa Aesar |
| 電話番号: |
400-6106006 |
| 電子メール: |
saleschina@alfa-asia.com |
|
| 化学名: | フッ化ケイ素酸 | | 英語化学名: | Hexafluorosilicic acid | | 别名: | FLUOROSILICIC ACID, 20-25% SOLUTION;Fluorosilicic acid, Hexafluorosilicic acid, Hydrogen hexafluorosilicate;FLUOROSILIC ACID;CORTICOTROPIN A;Hexafluorosilicic acid, 35% w/w aq. soln.;Hexafluorosilicic acid, 23% w/w aq. soln.;Silicofluoric acid: (Fluorosilicic acid);FLUOROSILICIC ACID, 25% in water | | CAS番号: | 16961-83-4 | | 分子式: | F6H2Si | | 分子量: | 144.09 | | EINECS: | 241-034-8 | | カテゴリ情報: | Fluoride;peptide;Acids;Electronic Chemicals;Micro/Nanoelectronics;Inorganics | | Mol File: | 16961-83-4.mol | 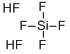 |
| 沸点 | 108-109°C | | 比重(密度) | 1.22 g/mL at 20 °C (lit.)
1.31 g/mL at 25 °C | | 蒸気圧 | 23hPa at 19.85℃ | | 屈折率 | 1.3500 | | 闪点 | 108-109°C | | 貯蔵温度 | −20°C | | 溶解性 | H2O: 1 mg/mL, clear, colorless | | 外見 | Liquid | | 酸解離定数(Pka) | 1.83[at 20 ℃] | | 比重 | 1.38 (40%) | | 色 | Clear colorless | | 水溶解度 | Miscible with water. | | Hydrolytic Sensitivity | 0: forms stable aqueous solutions | | Merck | 14,4182 | | 暴露限界値 | ACGIH: TWA 2.5 mg/m3
NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 | | 安定性: | Stable in aqueous solution. | | InChIKey | AUJBMDCSBIPDEH-UHFFFAOYSA-N | | CAS データベース | 16961-83-4(CAS DataBase Reference) | | EPAの化学物質情報 | Fluosilicic acid (16961-83-4) |
| | フッ化ケイ素酸 Usage And Synthesis |
| 外観 | 無色澄明の液体 | | 溶解性 | 水に混和する。 | | 解説 | フッ化ケイ素酸二水和物は無色の結晶。融点19℃。四水和物H2SiF6・4H2Oも知られている。融点約0℃。水溶液は硫酸と同程度の強い二塩基酸。電離度(25℃)は、一規定では53%、0.1規定では76%。水溶液はわずかに2HFとSiF4に解離する。防腐剤、製紙工業に用いられる。また、鉛の電解精製の電解液となる。ナトリウム塩は、氷晶石やフッ化ナトリウムの製造、うわぐすり、ガラス、陶磁器の乳白剤などに用いられる。刺激臭をもち、きわめて有毒。 小学館 日本大百科全書(ニッポニカ) ) | | 用途 | 合成原料、鉛の電解精錬、メッキ材料、金属表面処理剤。 | | 用途 | 亜鉛、すず、鉛等の電解精錬、繊維の媒染剤 | | 製造 | 四フッ化ケイ素を水と反応させると、コロイド状のケイ酸とともに生じる。あるいはフッ化水素酸に二酸化ケイ素を溶かして得られる。 3SiF4+3H2O→2H2SiF6+H2SiO3これにフッ化水素酸を加えてケイ酸を溶かすと多量に得られる。 | | 説明 | Hexafluorosilicic acid is a kind of inorganic acid. It is majorly used for the fluoridation of water in United State to minimize the incidence of dental caries and dental fluorosis. For chemical synthesis, it is majorly used for the manufacturing of aluminum fluoride and cryolite as well as many kinds of hexafluorosilicate salts. It can also be used for the production of silicon and silicon dioxide. It can also be used as an electrolyte in the Betts electrolytic process for refining lead. It is also a specialized reagent in organic synthesis for cleaving Si–O bonds of silyl ethers. | | 化学的特性 | Fluosilicic acid,H2SiF6, also known as hydrofluorosilicic acid,is a colorless liquid that is soluble in water. It is highly corrosive and toxic,attacking glass and stoneware. Fluosilicic acid is used in water fluoridation, electroplating, and in manufacturing enamels and cement. | | 化学的特性 | Fluorosilicic acid is a transparent, colorless
fuming liquid. | | 物理的性質 | d 1.220 g cm?3 for a 25% aq solution. | | 使用 | A fluoride source with both protic and Lewis acid properties providing efficient cleavage of silicon–oxygen bonds, e.g. silyl ether
deprotection. | | 使用 | Hexafluorosilicic acid is commonly used as a source of fluoride. It is converted to a variety of useful hexafluorosilicate salts. It is also used as an electrolyte in the Betts electrolytic process for refining lead. It is an important organic reagent for cleaving Si-O bonds of silyl ethers. Further, it is used as wood a preservation agent and also used in surface modification of calcium carbonate. | | 使用 | A 1-2% solution is used widely for sterilizing equipment in brewing and bottling establishments. Other concentrations are used in the electrolytic refining of lead, in electroplating, for hardening cement, crumbling lime or brick work, for the removal of lime from hides during the tanning process, to remove molds, as preservative for timber. | | 一般的な説明 | A colorless fuming liquid with a penetrating pungent odor. Corrosive to metals and tissue. Both the fumes and very short contact with the liquid can cause severe and painful burns. Used in water fluoridation, in hardening cement and ceramics, as a wood preservative. | | 空気と水の反応 | Fumes in air. Soluble in water with release of heat and corrosive fumes. | | 反応プロフィール | Hexafluorosilicic acid can react with strong acids (such as sulfuric acid) to release fumes of toxic hydrogen fluoride. Attacks glass and materials containing silica. Reacts exothermically with chemical bases (examples: amines, amides, inorganic hydroxides). Reacts with active metals, including iron and aluminum to dissolve the metal and liberate hydrogen and/or toxic gases. Can initiate polymerization in certain alkenes. Reacts with cyanide salts and compounds to release gaseous hydrogen cyanide. Flammable and/or toxic gases are also often generated by reactions with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and weak or strong reducing agents. Additional gas-generating reactions may occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and carbonates. Can catalyze (increase the rate of) chemical reactions. Decomposes when heated to the boiling point to produce very toxic and corrosive hydrogen fluoride gas. | | 危険性 | Extremely corrosive by skin contact and
inhalation. | | 健康ハザード | Inhalation of vapor produces severe corrosive effect on mucous membrane. Ingestion causes severe burns of mouth and stomach. Contact with liquid or vapor causes severe burns of eyes and skin. | | 火災危険 | Special Hazards of Combustion Products: Irritating fumes of hydrogen fluoride may form in fire. | | 燃焼性と爆発性 | Not classified | | 工業用途 | Hydrofluorosilicic acid (H2SiF6) is a colorless to light brown liquid. It is also manufactured
from calcium fluoride or other fluoride-containing products. Hydrofluorosilic acid
is a strong depressant for many silicates during flotation of a number of oxidic minerals.
It is used for gangue depression during flotation of tin, columbite and tantalite. | | 安全性プロファイル | Poison by subcutaneous route. A corrosive irritant to sktn, eyes, and mucous membranes. Will react with water or steam to produce toxic and corrosive fumes. When heated to decomposition it emits toxic fumes of F-. See also FLUORIDES. | | 職業ばく露 | A solution of fluorosilicic acid is used
for sterilization in the brewing and bottling industry, elec trolytic refining of lead; electroplating, hardening cement;
removing mold, and others. | | 応急処置 | this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at leastFluosilicic acid 134515 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. If victim is conscious, administer water or milk. Do not induce vomiting.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.Note to physician: Inject intravenously 10 mL of 10% calcium gluconate solution. Gastric lavage with lime water of1% calcium chloride. | | 貯蔵 | (1) Color Code—White: Corrosive or ContactHazard; Store separately in a corrosion-resistant location.(2) Color Code—Blue: Health Hazard/Poison: Store in asecure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Store in a cool, dry area that is well ventilated. Protect fromdamage. Avoid acids. Concentrated solution can be storedin glass but lead is preferred. | | 輸送方法 | UN1778 Fluorosilicic acid, Hazard class: 8;
Labels: 8-Corrosive material. | | 不和合性 | The aqueous solution is a strong acid.
Reacts with water or steam to produce toxic and corrosive
fumes of hydrogen fluoride. Incompatible, and may react violently with: bases, aliphatic amines; alkanolamines,
alkylene oxides; aromatic amines; amides, ammonia,
ammonium hydroxide; calcium oxide; epichlorohydrin, iso cyanates, oleum, organic anhydrides; sulfuric acid; strong
oxidizers; vinyl acetate; water. Attacks glass, concrete, and
ceramics. The anhydrous form dissociates almost instantly
into silicon tetrafluoride and hydrogen fluoride. | | 廃棄物の処理 | Add slowly to a large amount
of soda ash in solution. Discharge to sewer with large
volumes of water | | 参考文献 | Robinson, Tim. "Innovative Processes in Electrometallurgy." Innovative Process Development in Metallurgical Industry. Springer International Publishing, 2016. 385- 392.
Sarawade, Pradip B., et al. "Recovery of high surface area mesoporous silica from waste hexafluorosilicic acid (H 2 SiF 6) of fertilizer industry." Journal of hazardous materials 173.1 (2010): 576-580.
Kauffman, Joel M. "Water fluoridation: a review of recent research and actions." Journal of American Physicians and Surgeons 10.2 (2005): 38.
Krot, V. V., et al. "ChemInform Abstract: Preparation of Amorphous Silicon Dioxide from Hexafluorosilicic Acid." Cheminform 23.48(1992):no-no.
Zorya, L., and V. Krot. "Method of high-purity silica production from hexafluorosilicic acid." Reaction Kinetics & Catalysis Letters 50.1-2(1993):349-354. |
|