|
ChemicalBook Optimization Suppliers |
|
| 融点 | 86-89 °C (lit.) | | 沸点 | 125 °C (lit.) | | 比重(密度) | 0.87 g/mL at 20 °C(lit.) | | 蒸気圧 | 60-710Pa at 25℃ | | 屈折率 | n20/D 1.46 | | 闪点 | 73 °F | | 貯蔵温度 | Store below +30°C. | | 溶解性 | very sol nearly all common organic solvents such as
THF, methylene chloride, and DMF. | | 外見 | white solid | | 色 | white | | 比重 | 1.225 | | 水溶解度 | Soluble in chloroform and ethyl acetate. Insoluble in water. | | Hydrolytic Sensitivity | 7: reacts slowly with moisture/water | | Sensitive | Moisture Sensitive | | BRN | 505999 | | 暴露限界値 | ACGIH: TWA 20 ppm
OSHA: Ceiling 300 ppm; TWA 200 ppm
NIOSH: IDLH 500 ppm; TWA 100 ppm(375 mg/m3); STEL 150 ppm(560 mg/m3) | | InChIKey | BCNZYOJHNLTNEZ-UHFFFAOYSA-N | | LogP | 2.5 at 20℃ | | CAS データベース | 18162-48-6(CAS DataBase Reference) | | NISTの化学物質情報 | Silane, chloro(1,1-dimethylethyl)dimethyl-(18162-48-6) | | EPAの化学物質情報 | Silane, chloro(1,1-dimethylethyl)dimethyl- (18162-48-6) |
| 主な危険性 | C,F,T,Xn | | Rフレーズ | 22-35-40-34-10-19-11-67-65-63-48/20-20/21/22-45-23/24/25-37-36/37/38 | | Sフレーズ | 26-36/37/39-46-62-45-25-16-28-27-33-29-53-36/37 | | RIDADR | UN 2925 4.1/PG 2 | | WGK Germany | 2 | | RTECS 番号 | VV2000000 | | F | 10-21 | | Hazard Note | Flammable/Corrosive/Moisture Sensitive | | TSCA | Yes | | 国連危険物分類 | 4.1 | | 容器等級 | III | | HSコード | 29310095 | | 毒性 | mouse,LDLo,intraperitoneal,1gm/kg (1000mg/kg),IMMUNOLOGICAL INCLUDING ALLERGIC: DECREASED IMMUNE RESPONSE,Personal Communication from G.D. Stoner, Dept. of Community Medicine, School of Medicine, Univ. of California, La Jolla, CA 92037, May 25, 1975Vol. 27MAY1975, |
| | tert-ブチルジメチルクロロシラン Usage And Synthesis |
| 外観 | 白色~ほとんど白色粉末~結晶 | | 用途 | ガスクロマトシリル化用。
| | 化学的特性 | tert-Butyldimethylsilyl chloride is a colorless or white solid that is soluble in many organic solvents but reacts with water and alcohols.It is an organosilicon compound that can be used as a versatile protecting reagent for alcohols, amines, amides, and various carboxylic acids. | | 使用 | tert-Butyldimethylchlorosilane is used to protect hydroxyl group in organic synthesis. It finds application in the synthesis of prostaglandin. It is also used as an auxiliary material for hypolipaemics such as lovastatin and simvastatin. It plays an important role in the preparation of isoxazolines N-oxides from alpha-bromonitroalkanes. It acts as a versatile protecting reagent for amines, amides and alcohols. | | 製造方法 | tert-butyldimethylsilyl chloride (TBSCI) was synthesized by the reaction of tert-butyllithium with dichlorodimethylsilane.
The pentane solution of dichlorodimethylsilane was cooled to 0°C, and the pentane solution of tert-butyllithium was added dropwise with stirring under nitrogen. The temperature was maintained at 0 °C, stirred for 1.5 h and then heated to 25 °C, and the reaction was continued for 48 h. Distillation, collect 125 ℃ (97.5kPa) fractions, stand to solidify, and obtain tert-butyldimethylsilyl chloride. Yield 70%. | | 主な応用 | Tert-butyldimethylsilyl chloride is a sterically hindered organosilicon protective agent, which is widely used in the synthesis of original drugs. It is used as a protective group for hydroxyl in the synthesis of ribonucleosides, and is also an oxidant and decyanide.
t-Butyldimethylchlorosilane is useful as an anion trapping reagent. For example, TBDMSCl was found to be an efficient trap of the lithio α-phenylthiocyclopropane anion.When dichlorothiophene was treated with 2 equiv of n-butyllithium followed by 2 equiv of TBDMSCl the di-TBDMS-thiophene was isolated. The lithium anions of primary (eq 1) and secondary (eq 2) nitriles were trapped with TBDMSCl to give C,N-disilyl- and Nsilylketenimines in excellent yields.
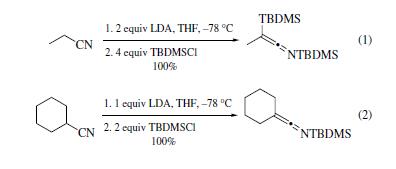
Silyl stannanes have been prepared by trapping tin anions with TBDMSCl or other silyl chlorides. Alkynes treated with silyl stannanes and catalytic tetrakis(triphenylphosphine)palladium(0) give cis-silyl stannylalkenes in good yields. | | 定義 | ChEBI: Tert-butyldimethylsilyl chloride is a silyl chloride consisting of a central silicon atom covalently bound to one chloro, one tert-butyl and two methyl groups. tert-Butyldimethylsilyl chloride is a derivatisation agent used in gas chromatography/mass spectrometry applications. It has a role as a chromatographic reagent. | | 反応性 | tert-Butyldimethylsilyl chloride reacts with alcohols in the presence of base to give tert-butyldimethylsilyl ethers:
(Me3C)Me2SiCl + ROH → (Me3C)Me2SiOR + HCl
These silyl ethers hydrolyze much more slowly than the trimethylsilyl ethers. | | 危険性 | Moderately toxic. | | 燃焼性と爆発性 | Flammable | | 純化方法 | Fractionally distil it at atmospheric pressure. [Sommer & Tyler J Am Chem Soc 76 1030 1954, Corey & Venkateswarlu J Am Chem Soc 94 6190 1972, Beilstein 4 IV 4076.] |
|