|
ChemicalBook Optimization Suppliers |
| 名前: |
tonsooh Gold |
| 電話番号: |
027-83238443 13343438265 |
| 電子メール: |
1569708153@qq.com |
| 名前: |
Alfa Aesar |
| 電話番号: |
400-6106006 |
| 電子メール: |
saleschina@alfa-asia.com |
|
| 化学名: | t-ブチルヒドロペルオキシド | | 英語化学名: | tert-Butyl hydroperoxide | | 别名: | 1,1-dimethylethyl-hydroperoxid;1,1-dimethylethylhydroperoxide;trigonoxa-75(czech);trigonoxa-w70;USP -800;T-HYDRO(R);TERT-BUTYL HYDROPEROXIDE;tert-Butyl hydroperoxide(content≤80%,with di-tert-butyl hydroperoxide and/or type A thinner)tert-Butyl hydrogen peroxide | | CAS番号: | 75-91-2 | | 分子式: | C4H10O2 | | 分子量: | 90.12 | | EINECS: | 200-915-7 | | カテゴリ情報: | Organic Peroxide;Synthetic Organic Chemistry;Organics | | Mol File: | 75-91-2.mol | 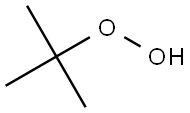 |
| 融点 | -2.8 °C | | 沸点 | 37 °C (15 mmHg) | | 比重(密度) | 0.937 g/mL at 20 °C | | 蒸気圧 | 62 mmHg at 45 °C | | 屈折率 | n20/D 1.403 | | 闪点 | 85 °F | | 貯蔵温度 | 2-8°C | | 酸解離定数(Pka) | pK1: 12.80 (25°C) | | 外見 | Liquid | | 色 | Clear colorless | | 水溶解度 | Miscible | | Merck | 14,1570 | | BRN | 1098280 | | 暴露限界値 | No exposure limit is set. On the basis
of its irritant properties a ceiling limit of
1.2 mg/m3 (0.3 ppm) is recommended. | | 安定性: | Stable, but may explode if heated under confinement. Decomposition may be accelerated by traces of metals, molecular sieve or other contaminants. Incompatible with reducing agents, combustible material, acids. | | InChIKey | CIHOLLKRGTVIJN-UHFFFAOYSA-N | | LogP | 1.230 (est) | | CAS データベース | 75-91-2(CAS DataBase Reference) | | NISTの化学物質情報 | Tert-butyl hydroperoxide(75-91-2) | | EPAの化学物質情報 | tert-Butyl hydroperoxide (75-91-2) |
| | t-ブチルヒドロペルオキシド Usage And Synthesis |
| 外観 | 無色~ほとんど無色透明液体 | | 溶解性 | 水に可溶 ≧10 g/100 mL at 22℃。水、エタノール及びアセトンと任意の割合で混和する。 | | 解説 | t-ブチルヒドロペルオキシド,工業製品は,約70% 水溶液である.融点4.0~4.5 ℃,沸点35 ℃(2.7 kPa).d204 0.896.n20D 1.4007.有機溶媒に可溶.約75 ℃ まで安定であるが,常圧蒸留では爆発する.有機合成では,ヘキサカルボニルモリブデンなどの金属触媒の存在下,不飽和アルコールの不斉エポキシ化,シリルエノールエーテルの酸化開裂,アリル位の酸化などに広く用いられている.重合触媒,硬化剤として工業的に利用される.
森北出版「化学辞典(第2版) | | 用途 | 有機合成試薬。 | | 用途 | 樹脂製造用重合剤、硬化剤 | | 製造 | t-ブチルアルコールまたはイソブチレンを硫酸でスルホン化し,ついで過酸化水素と反応させて工業的に製造されているt-ブチルヒドロペルオキシド. | | 毒性 | LD50 406 mg/kg(ラット,経口). | | 説明 | tert-Butyl hydroperoxide (TBHP) is an organic peroxide widely
used in a variety of oxidation processes. | | 化学的特性 | tert-Butyl hydroperoxide (TBHP) is a water-white liquid
commonly commercially available as a 70% solution in
water; 80% solutions are also available. It is used to initiate
polymerization reactions and in organic syntheses to introduce peroxy groups into the molecule. TBHP vapor can
burn in the absence of air and may be flammable at either
elevated temperature or at reduced pressure. Fine mist/spray
may be combustible at temperatures below the normal flash
point. When evaporated, the residual liquid will concentrate
TBHP content and may reach an explosive concentration
(>90%). Closed containers may generate internal pressure
through the degradation of TBHP to oxygen . TBHP is a
highly reactive product. The three types of significant physical
hazards are flammability, thermal, and decomposition
due to contamination.
To minimize these hazards, avoid exposure to heat, fire, or
any condition that will concentrate the liquid material. Store
away from heat, sparks, open flames, foreign contaminants,
combustibles, and reducing agents. Inspect containers frequently
to identify bulges or leaks (7a, 125). | | 使用 | tert-Butyl hydroperoxide is used as an initiator for radical polymerization and in various oxidation process such as sharpless epoxidation. It is involved in osmium catalyzed vicinal hydroxylation of olefins under alkaline conditions. Furthermore, it is used in catalytic asymmetric oxidation of sulfides to sulfoxides using binaphthol as a chiral auxiliary and in the oxidation of dibenzothiophenes. It plays an important role for the introduction of peroxy groups in organic synthesis. | | 使用 | TBHP is an intermediate in the production of propylene
oxide and t-butyl alcohol from isobutane and propylene. It is
primarily used as an initiator and finishing catalyst in the
solution and emulsion polymerization methods for polystyrene
and polyacrylates. Other uses are for the polymerization
of vinyl chloride and vinyl acetate and as an oxidation
and sulfonation catalyst in bleaching and deodorizing operations.
It is a strong oxidant and reacts violently with
combustible and reducing materials, and metallic and sulfur
compounds. | | 調製方法 | TBHP is produced by the liquid-phase reaction of isobutane
and molecular oxygen or by mixing equimolar amounts of
t-butyl alcohol and 30–50% hydrogen peroxide. TBHP can
also be prepared from t-butyl alcohol and 30% hydrogen
peroxide in the presence of sulfuric acid or by oxidation of
tert-butylmagnesium chloride. The manufacturing process of
TBHP is in a closed system. | | 定義 | ChEBI: Tert-butyl hydroperoxide is an alkyl hydroperoxide in which the alkyl group is tert-butyl. It is widely used in a variety of oxidation processes. It has a role as an antibacterial agent and an oxidising agent. | | 一般的な説明 | Watery odorless colorless liquid. Floats and mixes slowly with water. | | 空気と水の反応 | Water soluble. | | 反応プロフィール | Most alkyl monohydroperoxides are liquid. The explosivity of the lower members (e.g., methyl hydroperoxide, or possibly, traces of the dialkyl peroxides) decreasing with increasing chain length and branching [Bretherick 2nd ed. 1979 p. 10]. Though relatively stable, explosions have been caused by distillation to dryness [Milas, JACS 1946, 68, 205] or attempted distillation at atmospheric pressure [Castrantas 1965 p. 15]. | | 危険性 | Moderate fire risk. Oxidizer. | | 健康ハザード | tert-Butyl hydroperoxide is a strong irritant.Floyd and Stockinger (1958) observed thatdirect cutaneous application in rats did notcause immediate discomfort, but the delayedaction was severe. The symptoms were erythemaand edema within 2–3 days. Exposureto 500 mg in 24 hours produced asevere effect on rabbit skin, while a rinse of150 mg/min was severe to eyes.
It is moderately toxic; the effects aresomewhat similar to those of MEK peroxide.Symptoms from oral administration in ratswere weakness, shivering, and prostration.
LD50 value, intraperitoneal (rats): 87 mg/kg
LD50 value, oral (rats): 406 mg/kg. | | 燃焼性と爆発性 | tert-Butyl hydroperoxide is a flammable liquid and a highly reactive oxidizing agent.
Pure TBHP is shock sensitive and may explode on heating. Carbon dioxide or dry
chemical extinguishers should be used for fires involving tert-butyl hydroperoxide. | | 作用機序 | The general mechanism of transition metal-catalyzed oxidative Mannich reactions of N, N-dialkyl anilines with tert-butyl hydroperoxide (TBHP) as the oxidant consists of a rate-determining single electron transfer (SET) that is uniform from 4-methoxy- to 4-cyano-N, N-dimethylanilines. The tert-butylperoxy radical is the major oxidant in the rate-determining SET step that is followed by competing backward SET and irreversible heterolytic cleavage of the carbon–hydrogen bond at the α-position to nitrogen. A second SET completes the conversion of N, N-dimethylaniline to an iminium ion that is subsequently trapped by the nucleophilic solvent or the oxidant prior to the formation of the Mannich adduct[1].
Tert-butyl hydroperoxide could induce oxidative stress in liver mitochondria at low concentrations. The damaging effect of low concentrations of tBHP in the course of pyruvate oxidation in isolated liver mitochondria is caused by the opening of the nonspecific Ca2+-dependent cyclosporin A-sensitive pore in the inner mitochondrial membrane[2]. | | 安全性プロファイル | Moderately toxic by
ingestion and inhalation. A severe skin and
eye irritant. Mutation data reported. At
highest dosage levels, symptoms noted were
severe depression, incoordmation, and
cyanosis. Death was due to respiratory
arrest. Very dangerous fire hazard when
exposed to heat or flame, or by spontaneous
chemical reaction such as with reducing
materials. Moderately explosive; may
explode during distillation. Violent reaction
with traces of acid. Concentrated solutions
may ignite spontaneously on contact with
molecular sieve. Mixtures with transition
metal salts may react vigorously and release
oxygen. Forms an unstable solution with
1,2-dichloroethane. To fight fire, use alcohol
foam, CO2, dry chemical. When heated to
decomposition it emits acrid smoke and
fumes. See also PEROXIDES, ORGANIC. | | 発がん性 | A study performed to evaluate
the carcinogenicity of TBHP found it was not carcinogenic
when applied to the skin of mice at 16.6% of the peroxide 6
times a week for 45 weeks. However, if its application was
preceded by 0.05 mg of 4-nitroquinoline-1-oxide as a 0.25%
solution in benzene applied 20 times over 7 weeks followed
by TBHP (16.6% in benzene), then malignant skin tumors
appeared between days 390 and 405 of the experiment .
This supports the theory that peroxides are not complete
carcinogens, but may act as promoters . The effects of
TBHP on promotable and nonpromotable mouse epidermal
cell culture lines were reported by Muehlematter et al. . | | 貯蔵 | tert-butyl hydroperoxide should be stored in the dark at room temperature
(do not refrigerate) separately from oxidizable compounds, flammable substances,
and acids. Reactions involving this substance should be carried out behind a safety
shield. | | Toxicity evaluation | TBHP accelerates oxidation of glutathione and decreases the
metabolism of sodium hexobarbital in rat livers and is a strong
oxidation agent. | | 不和合性 | tert-Butyl hydroperoxide and concentrated aqueous solutions of TBHP react
violently with traces of acid and the salts of certain metals, including, in particular,
manganese, iron, and cobalt. Mixing anhydrous tert-butyl hydroperoxide with
organic and readily oxidized substances can cause ignition and explosion. TBHP can
initiate polymerization of certain olefins. | | 廃棄物の処理 | Excess tert-butyl hydroperoxide and waste material containing this substance should be placed in an
appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines. | | 参考文献 | [1] Maxim O, et al. Mechanistic Investigation of Oxidative Mannich Reaction with tert-Butyl Hydroperoxide. The Role of Transition Metal Salt. Journal of the American Chemical Society, 2013; 135: 1549–1557.
[2] Fedotcheva N, et al. Mechanism of induction of oxidative stress in liver mitochondria by low concentrations of tert-butyl hydroperoxide. Biochemistry (Moscow), 2013; 78: 75–79.
|
|