|
|
| | valdetamide Basic information |
| Product Name: | valdetamide | | Synonyms: | valdetamide;diethylallylacetamide;2,2-Diethyl-4-pentenamide;2,2-Diethyl-4-pentenoic amide;Epinoval;Novonal;2,2-diethylpent-4-enamide;2,2-diethyl-pent-4-enoic acid amide | | CAS: | 512-48-1 | | MF: | C9H17NO | | MW: | 155.24 | | EINECS: | 208-143-2 | | Product Categories: | | | Mol File: | 512-48-1.mol | 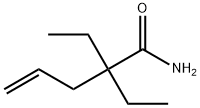 |
| | valdetamide Chemical Properties |
| Melting point | 75-76° | | Boiling point | 279.06°C (rough estimate) | | density | 0.9796 (rough estimate) | | refractive index | 1.4640 (estimate) | | pka | 16.59±0.50(Predicted) | | CAS DataBase Reference | 512-48-1 |
| | valdetamide Usage And Synthesis |
| Originator | Insomnia,ICN | | Definition | ChEBI: Valdetamide is a fatty amide. | | Manufacturing Process | 1 kg of diethylacetonitrile dissolved in 3 litres of dry ether is gradually mixed
in a reflex apparatus, while stirring and heating, with 400 g of potassium.
Thereupon evolution of hydrogen sets in and the potassium passes slowly into
solution. After three hours any small amount of potassium, which may have
remained unattacked is separated, and the solution is mixed with 1200 g of
allyl bromide. The ether begins to boil and potassium bromide separates. The
mass is heated for another hour, filtered, and the filtrate, after having distilled
off the ether, is distilled in a vacuum. Thus diethylallylacetonitrile, distilling
over at 78°C/9mm is obtained. By subsequent saponification with alcoholic
potash the diethylallylacetamide is obtained. MP: 74°C. | | Therapeutic Function | Hypnotic | | Safety Profile | Poison to humans by
ingestion. An experimental poison by
ingestion, rectal, and intraperitoneal routes.
Human systemic effects by ingestion:
muscle spasms, cardiac arrhythmias, and
respiratory depression. When heated to
decomposition it emits toxic fumes of NOx |
| | valdetamide Preparation Products And Raw materials |
|