| Company Name: |
J & K SCIENTIFIC LTD.
|
| Tel: |
010-82848833 400-666-7788 |
| Email: |
jkinfo@jkchemical.com |
| Products Intro: |
Product Name:Caroxazone
CAS:18464-39-6
Package:100Mg,10Mg
|
| Company Name: |
Shanghai Hekang Biotechnology Co., Ltd.
|
| Tel: |
18939837085 |
| Email: |
youchemicals@gmail.com |
| Products Intro: |
Product Name:Caroxazone
CAS:18464-39-6
Purity:98+% Package:1g, 5g, 10g, 25g, 100g, 1kg, 1000kg Remarks:C8377
|
|
| | caroxazone Basic information |
| Product Name: | caroxazone | | Synonyms: | caroxazone;2-Oxo-2H-1,3-benzoxazine-3(4H)-acetamide;F.I.-6654;FI-6654;Surodil;Timostenil;2H-1,3-Benzoxazine-3(4H)-acetamide, 2-oxo- | | CAS: | 18464-39-6 | | MF: | C10H10N2O3 | | MW: | 206.2 | | EINECS: | 2423451 | | Product Categories: | | | Mol File: | 18464-39-6.mol | 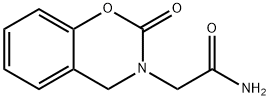 |
| | caroxazone Chemical Properties |
| Melting point | 203-205° | | Boiling point | 435.1±45.0 °C(Predicted) | | density | 1.345±0.06 g/cm3(Predicted) | | pka | 15.73±0.40(Predicted) |
| RIDADR | 3249 | | HazardClass | 6.1(b) | | PackingGroup | III |
| | caroxazone Usage And Synthesis |
| Originator | Timostenil,Farmitalia,Italy,1975 | | Uses | Caroxazone acts as an irreversible, nonselective MAO inhibitor, displaying antidepressant characteristics. | | Definition | ChEBI: Caroxazone is a benzoxazine. | | Manufacturing Process | 37.9 g of ethyl glycinate hydrochloride were dissolved in 400 cc of ethanol
and 33.5 g of salicylic aldehyde were added. It is refluxed for half an hour and
cooled. 38 cc of triethylamine and 25 g of Raney nickel are then added where
after hydrogenation is carried out at room temperature and under
atmospheric pressure. After hydrogen adsorption was complete, the mixture
was filtered and the alcohol evaporated off. The residue was taken up with
acidified water, extracted with ether to eliminate part of the by-products,
consisting mainly of o-cresol, then made alkaline with ammonia and extracted
with ethyl acetate. The solvent was removed in vacuo and the residue
crystallized from ether/petroleum ether. 36.7 g of o-hydroxybenzylaminoacetic
acid ethyl ester melting at 47°C are obtained.
20 g of this compound were dissolved in 100 cc of tetrahydrofuran and 100 cc
of a 30% solution of phosgene in tetrahydrofuran solution were added. After
one night at room temperature, the reaction mixture was dried, taken up with
150 cc of anhydrous pyridine and allowed to stand overnight. The pyridine
was then removed in vacuo and the residue dissolved in benzol was washed
several times with water and chromatographed over 250 g of alumina. Elution
with benzene/petroleum ether yielded 16 g of 4H-3-carboethoxymethyl-1,3-
benzoxazine-2-one, melting at 90°-91°C.
5 g of this last compound were dissolved in 120 cc of absolute ethanol and
saturated with NH3 at 0°C. It was allowed to stand overnight where after 1.5
g of 4H-3-carboxamidomethyl-1,3-andenzoxazine-2-one, melting at 205°C,
were obtained. By evaporation from the mother liquors further quantities of
the same product were obtained. | | Therapeutic Function | Antidepressant |
| | caroxazone Preparation Products And Raw materials |
|