| Company Name: |
Hangzhou Peptidego Biotech Co.,Ltd.
|
| Tel: |
0571-87213919 |
| Email: |
eric@peptidego.com |
| Products Intro: |
Product Name:Nafarelin Acetate
CAS:76932-60-0
Purity:95% or 98% Package:1mg;5mg;10mg;50mg;100mg,1g or according to customer's detail requirement.
|
| Company Name: |
Shanghai Hongye Biotechnology Co. Ltd
|
| Tel: |
400-9205774 |
| Email: |
sales@glpbio.cn |
| Products Intro: |
Product Name:Nafarelin (acetate)
CAS:76932-60-0
Purity:>98% Package:1mg;10mg;50mg;100mg;
|
|
| | Nafarelin acetate Basic information |
| Product Name: | Nafarelin acetate | | Synonyms: | Luteinizing hormone-releasing factor (pig), 6-[3-(2-naphthalenyl)-D-alanine]-, acetate (salt);Nafarelin acetate(76932-56-4 free base);Nafarelin acetate(76932564 free base),Nafarelin acetate(76932 56 4 free base);Luteinizing hormone-releasing factor (swine),6-[3-(2-naphthalenyl)-D-alanine]-, acetate (salt) | | CAS: | 76932-60-0 | | MF: | C68H87N17O15 | | MW: | 1382.55 | | EINECS: | | | Product Categories: | | | Mol File: | 76932-60-0.mol | 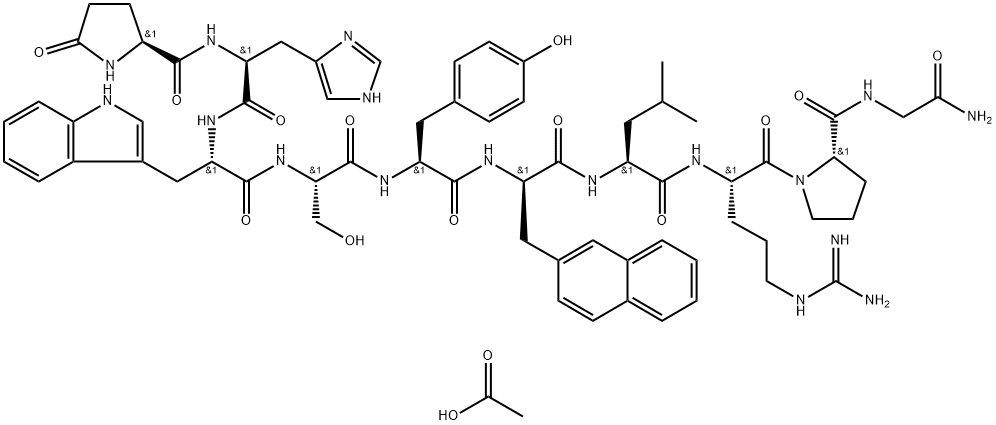 |
| | Nafarelin acetate Chemical Properties |
| storage temp. | Store at -20°C | | solubility | DMSO: Slightly Soluble; Methanol: Slightly Soluble; Water: Slightly Soluble | | form | A solid |
| | Nafarelin acetate Usage And Synthesis |
| Description | Nafarelin is an agonist of gonadotropin-releasing hormone (GNRH). It is a long-acting agent that, after an initial increase in sex hormone levels, decreases the level of circulating gonadotropins and sex hormones. In vivo, nafarelin (0.5-2.0 μg/kg, s.c.) reduces plasma levels of luteinizing hormone and testosterone (Item Nos. 15645 | ISO60154) as well as testicular volume, sperm count, sperm motility, and duration of ejaculation in male dogs. Nafarelin (32 μg/animal per day) inhibits estrus in female beagle dogs. It also reduces the volume of endometrial tissue in a rat model of endometriosis. Formulations containing nafarelin have been used in the treatment of endometriosis and central precocious puberty. | | Originator | Nafarelin Acetate,Bachem AG | | Definition | ChEBI: Nafarelin acetate is an acetate salt obtained by reaction of nafarelin with one equivalent of acetic acid. It has a role as a gonadotropin releasing hormone agonist and an anti-estrogen. It contains a nafarelin. | | Manufacturing Process | In the reaction vessel of a Beckman 990 Peptide Synthesizer was placed 0.8 g
(0.8 mmol) of benzhydrylamino-polystyrene-divinylbenzene resin (Lab
Systems, Inc.) as described by Rivaille, supra. Amino acids were added
sequentially to this resin by means of the usual methods of Boc-strategy of
peptide synthesis on above copolymer.
The resin was coupled sequentially with a 2.5 molar excess of each protected amino acid and DCC. Thus, the resin was treated during successive coupling
cycles with 0.433 g Boc-Gly-OH, 0.432 g Boc-Pro-OH, 0.857 g Boc-Arg(Tosyl)-
OH, 0.462 g Boc-Leu-OH, 0,504 g Boc-3-(2-naphthyl)-D-alanine and 0.272 g
1-hydroxybenzotriazole, 0.724 g N-Boc, O-2-bromobenzoyloxycarbonyl-L�tyrosine, 0.59 g Boc-Ser(Benzyl)-OH, 0.608 g Boc-Trp-OH, 0.654 g Boc�His(Tosyl)-OH and 0.524 g pyroglutamic acid. A coupling cycle for one amino
acid and completeness of the reaction is checked by the ninhydrin method of
E. Kaiser, et al., Anal. Biochem., 34, 595 (1970).
The resin was removed from the reaction vessel, washed with CH2Cl2, and
dried in vacuo to yield 2.0 g of protected polypeptide resin.
The polypeptide product was simultaneously removed from the resin and
completely deprotected by treatment with anhydrous liquid HF. A mixture of
2.0 g of protected polypeptide resin and 2 mL of anisole (scavenger) in a Kel�F reaction vessel was treated with 20 mL of redistilled (from CoF3) anhydrous
liquid HF at 0°C for 30 minutes. The HF was evaporated under vacuum and
the residue of (pyro)-Glu-His-Trp-Ser-Tyr-3-(2-naphthyl)-D-alanyl-Leu-Arg�Pro-Gly-NH2,as its HF salt, was washed with ether. The residue was then
extracted with glacial acetic acid. The acetic acid extract was lyophilized to
yield 0.8 g of crude material. The crude polypeptide was loaded on a 4x40
cm. Amberlite XAD-4 column (polystyrene-4% divinylbenzene copolymer) and
eluted with a concave gradient from water (0.5 L) to ethanol (1 L). The tubes
containing fractions from effluent volume 690 mL to 1,470 mL were pooled
and stripped to dryness to yield 490 mg of partially purified polypeptide.
A 150 mg sample of the partially purified product was subjected to partition
chromatography on a 3 times 50 cm. column of Sephadex G-25 using the
solvent system 1-butanol/toluene/acetic acid/water containing 1.5% pyridine
in the ratios 10:15:12:18. The pure fractions were pooled on the basis of thin
layer chromatography (silica gel; BuOH/H2O/HOAc/EtOAc; 1:1:1:1) and HPLC
(5 micron, reverse phase, octadecylsilyl packing; 40% 0.03 M NH4OAc/60%
acetonitrile). The desired product came off the column in fractions from
effluent volume 1,000 mL to 1,400 mL (Rf 0.1). The pure fractions were
pooled, stripped to dryness, taken up in H2O, and lyophilized to yield 57 mg of
pure pyro-glutamyl-histidyl-tryptophylseryl-tyrosyl-3-(2-naphthyl)-D-alanyl�leucyl-arginylprolyl-glycinamide, as its acetic acid addition salt, [α]D25-27.4°
(c 0.9, HOAc), m.p. 185°-193°C (dec.). | | Therapeutic Function | Gonadotropic |
| | Nafarelin acetate Preparation Products And Raw materials |
|