|
ChemicalBook Optimization Suppliers |
|
| 化学名: | 二酸化マンガン | | 英語化学名: | Manganese dioxide | | 别名: | Manganese(IV) oxide, -100 mesh, 99+%;Manganese(IV) oxide, average particle size 2 μm, 99+%;Manganese(IV) oxide, powder, 80-85%;MANGANESE PEROXIDE;MANGANESE SUPEROXIDE;MANGANESE (IV) DIOXIDE;MANGANESE(IV) OXIDE ACTIVATED;MANGANESE(IV) OXIDE ON CARRIER | | CAS番号: | 1313-13-9 | | 分子式: | MnO2 | | 分子量: | 86.94 | | EINECS: | 215-202-6 | | カテゴリ情報: | metal oxide;Inorganics | | Mol File: | 1313-13-9.mol | 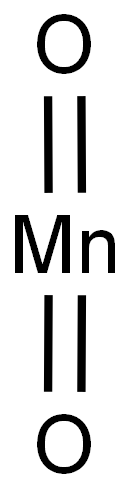 |
| 融点 | 535 °C (dec.) (lit.) | | 比重(密度) | 5.02 | | 蒸気圧 | 0-0Pa at 25℃ | | 貯蔵温度 | Store below +30°C. | | 溶解性 | <0.001g/l insoluble | | 外見 | powder | | 色 | gray | | 比重 | 5.026 | | 水溶解度 | insoluble | | Merck | 14,5730 | | 暴露限界値 | ACGIH: TWA 0.02 mg/m3; TWA 0.1 mg/m3
OSHA: Ceiling 5 mg/m3
NIOSH: IDLH 500 mg/m3; TWA 1 mg/m3; STEL 3 mg/m3 | | 安定性: | Stable. Incompatible with strong acids, strong reducing agents, organic materials. | | InChIKey | NUJOXMJBOLGQSY-UHFFFAOYSA-N | | CAS データベース | 1313-13-9(CAS DataBase Reference) | | NISTの化学物質情報 | manganese(IV) dioxide(1313-13-9) | | EPAの化学物質情報 | Manganese dioxide (1313-13-9) |
| 主な危険性 | Xn | | Rフレーズ | 20/22 | | Sフレーズ | 25 | | RIDADR | 3137 | | WGK Germany | 1 | | RTECS 番号 | OP0350000 | | TSCA | Yes | | HSコード | 2820 10 00 | | 容器等級 | III | | 毒性 | LD50 orally in rats: >40 mmole/kg (Holbrook) |
| | 二酸化マンガン Usage And Synthesis |
| 外観 | 黒色~黒褐色, 塊又は粉末 | | 定義 | 本品は、次の化学式で表される無機化合物である。 | | 溶解性 | 塩酸及び還元剤(しゅう酸等)を含む無機酸に溶けやすく、水にほとんど溶けない。還元剤を共存させた酸に溶け、水、エタノール及びアセトンにほとんど溶けない。 | | 解説 | 二酸化マンガン,にさんかまんがん.酸化マンガン(Ⅳ)ともいう.灰色~灰黒色の粉末.天然にはパイロルース鉱として産出する.実験室では,硝酸マンガン(Ⅱ)を空気中で約530 ℃ に加熱分解酸化してつくる.過マンガン酸アルカリを塩基性水溶液中で還元すると水和酸化物の形で生成する.正方晶系,ルチル型構造.密度5.03 g cm-3.比電気伝導率0.16 Ω-1 cm-1(0 ℃).水に不溶.空気中で安定であるが,加熱すると酸素を放って分解する.酸化作用が強く,濃塩酸に溶けて塩素を発する(塩素の製法).また,過酸化水素の酸性水溶液から酸素を発生して MnⅡとなる.過酸化水素水の分解,塩素酸カリウムの熱分解の触媒,金属マンガンおよびマンガン塩の原料,マンガン鋼,乾電池,マッチ,塗料,うわぐすり,ガラスの着色剤,媒染剤などに用いられる. | | 用途 | 水の除鉄、脱硝触媒。 | | 用途 | 酸素発生用、酸化剤、マンガン化合物製造原料。 | | 用途 | 高純度金属酸化物、触媒。 | | 用途 | 有機合成用(酸化剤)、乾電池材料、マンガン化合物製造原料。 | | 用途 | かなりの導電性がある。アルカリ性でマンガン(Ⅱ)化合物の酸化またはマンガン(Ⅶ)化合物の還元により、水和物MnO2・2H2Oが沈殿する。酸化剤となる。γ型が乾電池の減極剤に用いられる。マッチ、花火の材料となり、ガラスの青色を消すのに、また乾性油の製造などに用いる。実験室で酸素発生の触媒、有機化学の分野でアルコールの酸化などに用いる。 | | 化粧品の成分用途 | 酸化防止剤 | | 使用上の注意 | 純度は金属ベースで差数法によって算出したもので、重量又は容量分析等の化学的方法によるものではありません。使用目的により、正確な含量が必要な場合は、それらの方法によって測定する必要があります。 | | 説明 | Manganese dioxide is a black crystalline solid. Molecular weight= 86.94; Freezing/Melting point=(decomposes)=53℃. Hazard Identification (based on NFPA-704 M Rating System): Health 3, Flammability 0, Reactivity×(Oxidizer). Insoluble in water. | | 化学的特性 | Manganese dioxide is a black crystalline solid
or powder. | | 物理的性質 | Black tetragonal crystals; density 5.08 g/cm3; Moh’s hardness 6.3; decomposes at 535°C; insoluble in water. | | 使用 | Manganese(IV) oxide is the most important ore of manganese from which the metal is mostly manufactured. The oxide occurs in nature as the mineral pyrolusite as heavy gray lumps, or black when powdered.
The mineral is used to produce manganese metal, most manganese salts, and also manganese steel and other alloys. The metallurgical applications of manganese(IV) oxide mainly involve making ferromanganese and special manganese alloys. Another important application of manganese(IV) oxide is in manufacturing dry-cell batteries and alkaline cells. The oxide also is a colorant in brick, tile, porcelain and glass; a drier for paints and varnishes; a 552 MANGANESE(IV) OXIDEpreparation for printing and dyeing textiles; a curing agent for polysulfide rubbers; an adsorbent for hydrogen sulfide and sulfur dioxide; an oxidizing agent in many organic syntheses such as quinone and hydroquinone; and a catalyst in laboratory preparation of oxygen from potassium chlorate. Manganese(IV) oxide also is used to make welding rods and fluxes, and ceramic magnets (ferrites); and is an additive to fertilizers. | | 使用 | The mineral is the source of manganese and all its Compounds; largely used in manufacture of manganese steel; oxidizer; in alkaline batteries (dry cells); for making amethyst glass, decolorizing glass; painting on porcelain, faience and majolica. The ppt is used in electrotechnics, pigments, browning gun barrels, drier for paints and varnishes, printing and dyeing textiles. | | 使用 | As an oxidizing agent, source of metallic manganese, decolorizing glass, painting porcelain, as an analytical reagentManganese(IV) oxide is used as an oxidizing agent in organic synthesis such as oxidation of allylic/benzylic alcohols, as a textile dye, as a reducing agent, and as a component of dry cell batteries such as zinc-carbon battery and alkaline battery. It is also used in making pigments for glasses and ceramics, and drier for paints. Further it is used in the manufacture of manganese steel and several manganese derivatives including potassium permanganate, a powerful versatile oxidant. Catalytic hydration of nitriles to amides by flowing through manganese dioxide has been reported. Manganese dioxide supported on inorganic oxide can be used for oxidation of methylamine through CWAO (Catalytic Wet Air Oxidation). It has high potential as highly efficient and robust material for water oxidation reaction (WORs). | | 製造方法 | Pure manganese(IV) oxide (precipitate form) may be prepared by reducing permanganate ion with a manganous salt:
2KMnO4 + 3MnSO4 + 2H2O → 5MnO2 + K2SO4 + 2H2SO4
Manganese(IV) oxide can also be precipitated by oxidation of a manganese(II) salt using an oxidizing agent such as hypochlorite or peroxydisulphate:
Mn2+ + S2O82– + 2H2O → MnO2 + 2SO42– + 4H+
Manganese(IV) oxide may also be made by thermal decomposition of manganese(II) nitrate; or from roasting manganese(II) carbonate in air: Mn(NO3)2 → MnO2 + 2NO2
MnCO3 + ½ O2 → MnO2 + CO2
A highly active gamma-MnO2 can be produced by treating manganese(III) oxide with hot sulfuric acid:
Mn2O3 + H2SO4 → MnO2 + MnSO4 + H2O
Mn2O3 is derived from pyrolusite by heating the mineral at 600–800°C or reducing with powdered coal at 300°C. | | 一般的な説明 | Manganese(IV) oxide (MnO2) is an eco-friendly chemical having a high theoretical specific capacitance. | | 反応プロフィール | The stability of manganese dioxide is due primarily to its insolubility. It is, however, readily attacked by reducing agents in acid solution, for example oxidizing concentrated hydrochloric acid to chlorine. In hot concentrated alkali it dissolves to give a purple solution which contains an equimolar mixture of trivalent manganese, probably as (Mn(OH)6)3- and manganate(V), (MnO4)3-. Manganese dioxide is also one of the most active catalysts for the oxidation of carbon monoxide near room temperature. | | 危険性 | Oxidizing agent, may ignite organic materials. | | 燃焼性と爆発性 | Non flammable | | 工業用途 | Manganese dioxide (MnO2) is soluble in water and HNO3 and soluble in HCl. It occurs in nature as the blue-black mineral pyrolusite. In glass, manganese dioxide is used as a colorant and decolorizer.
The major use of manganese oxides is an ore of manganese for the manufacturing of steel; manganese serves to increase the hardness and decrease the brittleness of steel. Another important use of manganese oxides is as the cathode material of common zinc/carbon and alkaline batteries (such as flashlight batteries). | | 安全性プロファイル | Poison by intravenous
and intratracheal routes. Moderately toxic by
subcutaneous route. Experimental
reproductive effects. A powerful oxidizer.
Flammable by chemical reaction. It must not
be heated or rubbed in contact with easily
oxilzable matter. Violent thermite reaction
when heated with aluminum. Potentially explosive reaction with hydrogen peroxide,
peroxomonosulfuric acid, chlorates + heat,
anilinium perchlorate. Ignition on contact
with hydrogen sulfide. Violent reaction with
oxidizers, potassium azide (when warmed),
diboron tetrafluoride, Incandescent reaction
with calcium hydride, chlorine trifluoride,
rubidium acetylide (at 350℃). Vigorous
reaction with hydroxylaminium chloride.
Incompatible with H202, H2SO j, Naz02.
Keep away from heat and flammable
materials. See also MANGANESE
COMPOUNDS. | | 職業ばく露 | Manganese dioxide is used as depolarizer for dry cell batteries, for production of manganese metal; as an oxidizing agent; laboratory reagent;
and in making pyrotechnics and matches; in dry cell
batteries. | | 応急処置 | If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if Manganese dioxide 1665 heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. | | 貯蔵 | Color Code—Yellow: Reactive Hazard (strong oxidizer); Store in a location separate from other materials, especially flammables and combustibles. Prior to working with this chemical you should be trained on its proper handling and storage. Manganese dioxide must be stored to avoid contact with heat and flammable materials and oxidizers (such as perchlorates, peroxides, permanganates, chlorates, and nitrates), since violent reactions occur. See also incompatibilities above. See OSHA Standard 1910.104 and NFPA 43A Code for the Storage of Liquid and Solid Oxidizers for detailed handling and storage regulations. | | 輸送方法 | UN1479 Oxidizing solid, n.o.s., Hazard Class:
5.1; Labels: 5.1-Oxidizer, Technical Name Required.
UN3137 (powder) Oxidizing solid, flammable, Hazard
Class: 5.1; Labels: 5.1-Oxidizer, 4.1 Flammable solid,
Technical Name Required. | | 不和合性 | A powerful oxidizer. Incompatible with
strong acids; reducing agents; combustible materials (such as
fuel and clothing; organic materials. Mixtures with calcium
hydride is a heat- and friction-sensitive explosive. Vigorous
reaction with hydrogen sulfide, diboron tetrafluoride; calcium hydride; chlorine trifluoride; hydrogen peroxide; hydroxyaluminum chloride; anilinium perchlorate. Decomposes
when heated above 553�C producing manganese(III)oxide
and oxygen, which increases fire hazard. Reacts violently
with aluminum (thermite reaction), potassium azide; rubidium acetylide; in the presence of hea | | 廃棄物の処理 | Generators of waste (equal to
or greater than 100 kg/mo) containing this contaminant,
EPA hazardous waste number N450, must conform to
USEPA regulations for storage, transportation, treatment,
and disposal of waste. Dispose of waste material as
hazardous waste using a licensed disposal contractor to an
approved landfill. Dispose of contents and container to
an approved waste disposal plant. Containers must be
disposed of properly by following package label directions
or by contacting your local or federal environmental
control agency, or by contacting your regional EPA
office. All federal, state, and local environmental regulations must be observed. Do not discharge into drains
or sewers |
|