|
|
| | MAGNESIUM CHLORATE Basic information |
| Product Name: | MAGNESIUM CHLORATE | | Synonyms: | MAGNESIUM CHLORATE;chloratesaltofmagnesium;chloricacid,magnesiumsalt;Magnesium dichlorate hydrate;magnesium chIorate;Chloric acid, MagnesiuMsalt (2:1);de-fol-ate;e-z-off | | CAS: | 10326-21-3 | | MF: | Cl2MgO6 | | MW: | 191.21 | | EINECS: | 233-711-1 | | Product Categories: | | | Mol File: | 10326-21-3.mol | 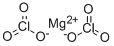 |
| | MAGNESIUM CHLORATE Chemical Properties |
| Melting point | ~35℃ [MER06] | | density | 1.80 [MER06] | | Water Solubility | mol/100mol H2O: 10.73 (0°C), 13.52 (25°C), 26.38 (93°C); solid phase Mg(ClO3)2 · 6H2O (0°C, 25°C), Mg(ClO3)2 · 2H2O (93°C) [KRU93] | | EPA Substance Registry System | Magnesium chlorate (10326-21-3) |
| RIDADR | 2723 | | HazardClass | 5.1 | | PackingGroup | II | | Toxicity | guinea pig,LDLo,oral,1500mg/kg (1500mg/kg),Meditsinskii Zhurnal Uzbekistana. Vol. (9), Pg. 26, 1959. |
| | MAGNESIUM CHLORATE Usage And Synthesis |
| Description | Magnesium chlorate is white crystalline solid.Molecular weight= 191.21; Boiling point= 120℃;Freezing/Melting point= 35℃. Soluble in water (reaction). | | Chemical Properties | Magnesium chlorate is white crystalline solid. | | Chemical Properties | White powder; bitter taste. Very hygroscopic. Soluble in water; slightly soluble in alcohol. | | Uses | Magnesium Chlorate can used in Defoliant, desiccant. | | Reactions | Magnesium chlorate is a powerful oxidizer. There is
an explosive reaction with copper(I) sulfide, an incandescent
reaction with antimony(III) sulfide, arsenic(III)
sulfide, tin(II) sulfide, and tin(IV) sulfide. This salt is
incompatible with Al, As, C, Cu, MnO2, organic matter,
organic acids, phosphorus or sulfur. Mixtures with
ammonium salts, with powdered metals, silicon, sulfur,
or sulfides are readily ignited and potentially unstable,
even at room temperature.
A combination of finely divided aluminum and
magnesium chlorate can explode by heat, percussion,
or friction. Magnesium chlorate is readily available
commercially. | | General Description | White deliquescent crystals or powder. Soluble in water and denser than water. Poses a dangerous fire risk when in contact with organic materials or heat. May be irritating to skin, eyes and mucous membranes. Used to make other chemicals. | | Air & Water Reactions | Soluble in water. | | Reactivity Profile | MAGNESIUM CHLORATE is a powerful oxidizer. Explosive reaction with copper(I) sulfide. Incandescent reaction with antimony(III) sulfide, arsenic(III) sulfide, tin(II) sulfide, tin(IV) sulfide. Incompatible with Al, As, C, Cu, MnO2, organic matter, organic acids, P and S. [Lewis, 3rd Ed . 786] . Mixtures with ammonium salts, with powdered metals, silicon, sulfur, or sulfides are readily ignited and potentially explosive [Bretherick 1979. p. 806]. A combination of finely divided aluminum and MAGNESIUM CHLORATE can explode by heat, percussion, or friction [Mellor 2:310. 1946-47]. | | Hazard | Dangerous fire risk in contact with organic
materials, strong oxidizing agent. | | Health Hazard | Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution. | | Fire Hazard | These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard. | | Potential Exposure | Used as a drying agent and defoliant | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Note to physician: Treat for methemoglobinemia.Spectrophotometry may be required for precise determination of levels of methemoglobin in urine. | | storage | olor Code—Yellow: Reactive Hazard; Store in alocation separate from other materials, especially flam mables and combustibles. Prior to working with magnesiumchlorate you should be trained on its proper handling andstorage. Magnesium chlorate must be stored to avoid con tact with aluminum, arsenic, carbon, copper, phosphorus,sulfur, magnesium oxide, metal sulfides, fuels, and strongacids, since violent reactions occur. Store in tightly closed1638 Magnesium chloratecontainers in a cool, well-ventilated area away from flammable and combustible materials. See OSHA Standard1910.104 and NFPA 43A Code for the Storage of Liquidand Solid Oxidizers for detailed handling and storageregulations. | | Shipping | UN2723 Magnesium chlorate. Hazard Class: 5.1;
Labels: 5.1-Oxidizer, Technical Name Required | | Incompatibilities | A strong oxidizer. Potentially explosive.
Violent reactions with arsenic, carbon, charcoal, copper,
phosphorus, sulfur, magnesium oxide; metal sulfides (copper sulfide, arsenic sulfide, tin sulfide; fuels, and strong
acids. Reacts with moisture |
| | MAGNESIUM CHLORATE Preparation Products And Raw materials |
|