|
ChemicalBook Optimization Suppliers |
|
| 化学名: | トリアムシノロンアセトニド | | 英語化学名: | Triamcinolone acetonide | | 别名: | ARISTODERM;9ALPHA-FLUORO-11BETA,21-DIHYDROXY-16ALPHA,17-ISOPROPYLIDENEDIOXY-1,4-PREGNADIENE-3,20-DIONE;9ALPHA-FLUORO-16ALPHA-HYDROXYPREDNISOLONE 16ALPHA,17ALPHA-ACETONIDE;9ALPHA-FLUORO-11BETA,16ALPHA,17ALPHA,21-TETRAHYDROXY-1,4-PREGNADIENE-3,20-DIONE 16,17-ACETONIDE;9a-fluoro-11b,16a,17a,21-tetrahydroxy-1,4-pregnadiene-3,20-dione 16,17-acetonide;9a-fluoro-16a-hydroxyprednisolone 16a,17a-acetonide;ALPHA-FLUORO-11BETA,16ALPHA,17,21-TETRA-HYDROXYPREGNA-1,4-DIENE-3,20-DIONE;1,4-PREGNADIEN-9-ALPHA-FLUORO-11-BETA, 16-ALPHA, 17,21-TETROL-3,20-DIONE-16,17-ACETONIDE | | CAS番号: | 76-25-5 | | 分子式: | C24H31FO6 | | 分子量: | 434.5 | | EINECS: | 200-948-7 | | カテゴリ情報: | NASACORT;Steroid and Hormone;Biochemistry;Steroids;Steroids (Others);Intermediates & Fine Chemicals;Pharmaceuticals;76-25-5 | | Mol File: | 76-25-5.mol | 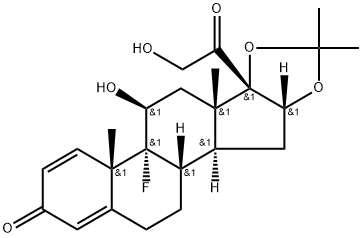 |
| 融点 | 274-278°C (dec.) | | 比旋光度 | D23 +109° (c = 0.75 in chloroform) | | 沸点 | 576.9±50.0 °C(Predicted) | | 比重(密度) | 1.1517 (estimate) | | 屈折率 | 1.5980 (estimate) | | 貯蔵温度 | Refrigerator | | 溶解性 | Practically insoluble in water, sparingly soluble in ethanol (96 per cent). | | 酸解離定数(Pka) | 12.87±0.10(Predicted) | | 色 | White to Off-White | | 水溶解度 | Soluble in DMSO or ethanol. Slightly soluble in water. | | Merck | 9596 | | BCS Class | 4 | | 安定性: | Combustible. Incompatible with strong oxidizing agents. | | InChIKey | YNDXUCZADRHECN-JNQJZLCISA-N | | NISTの化学物質情報 | Triamcinolone acetonide(76-25-5) | | EPAの化学物質情報 | Triamcinolone acetonide (76-25-5) |
| 主な危険性 | T | | Rフレーズ | 61 | | Sフレーズ | 53-45 | | WGK Germany | 3 | | RTECS 番号 | TU3920000 | | TSCA | Yes | | HSコード | 29372290 | | 毒性 | LD50 oral in mouse: 5gm/kg |
| | トリアムシノロンアセトニド Usage And Synthesis |
| 外観 | 白色~わずかにうすい褐色, 結晶性粉末~粉末 | | 溶解性 | アセトン,ジオキサンにやや可溶、メタノール,クロロホルムに難溶、水,エーテルにほとんど不溶。エタノールに溶け、水にほとんど溶けない。 | | 用途 | 薬理研究用。 | | 用途 | トリアムシノロン(Triamcinolone)は長時間作用型の合成コルチコステロイド(ステロイド剤)の一種。
トリアムシノロンのようなコルチコステロイドは、免疫系において重要な役割を果たすある種の化学物質の放出を阻害するよう細胞に作用し、炎症を和らげる。
トリアムシノロンは血液中の白血球の数を減少させる効果もあるとされる。これは白血球が異常に多く作られてしまう型の白血病の治療に有効である。また、免疫系が自らの体の組織を攻撃してしまう自己免疫疾患の治療にも用いられる。 | | 用途 | 副腎皮質ホルモン剤です。糖
質コルチコイドにフッ素を付加することで
で、糖質コルチコイド作用を強めています。 | | 効能 | 喘息治療薬, 抗炎症薬, 抗リウマチ薬, グルココルチコイド受容体作動薬 | | 商品名 | アフタシール (帝國製薬); アフタッチ (帝人ファーマ); ケナコルト−A (ブリストル・マイヤーズスクイブ); ケナコルト−A (ブリストル・マイヤーズスクイブ); マキュエイド (わかもと製薬); レダコート (アルフレッサファーマ); ワプロン (救急薬品工業) | | 説明 | Triamcinolone acetonide is a synthetic corticosteroid. It decreases cytokine levels, the firing rate of sensory neurons, and mechanical hypersensitivity in a rat spinal nerve ligation model when used at a dose of 1.5 mg/kg prior to and following surgery for three days. Triamcinolone acetonide also decreases outflow facility in a mouse model of steroid-induced glaucoma when 20 μl of a 40 mg/ml suspension is administered subconjunctivally. Formulations containing triamcinolone acetonide are used in the treatment of diabetic macular edema. | | 化学的特性 | White Solid | | Originator | Kenalog,Squibb,US,1958 | | 適応症 | Triamcinolone acetonide (Aristocort, Kenalog) is a synthetic fluorinated corticosteroid. | | 定義 | ChEBI: A synthetic glucocorticoid that is the 16,17-acetonide of triamcinolone. Used to treat various skin infections. | | Manufacturing Process | A solution of 250 mg of 9α-fluoro-11β,16α,17α,21-tetrahydroxy-1,4-
pregnadiene-3,20-dione in 70 ml of hot acetone and 7 drops of concentrated
hydrochloric acid is boiled for 3 minutes. After standing at room temperature
for 17 hours, the reaction mixture is poured into dilute sodium bicarbonate
and extracted with ethyl acetate. The extract is washed with saturated saline
solution, dried and evaporated to a colorless glass. The residue is crystallized
from acetone-petroleum ether to afford 166 mg of the acetonide, MP 270° to
274°C, decomposition, (with previous softening and browning). Three
recrystallizations from acetone-petroleum ether give 113 mg of 9α-fluoro-
11β,21-dihydroxy-16α,17α-isopropylidenedioxy-1,4-pregnadiene-3,20-dione,
MP 274° to 279°C, decomposition, (with previous softening and browning). | | brand name | Triamcinolone acetonide was sold under the brand name Kenalog among others. | | Therapeutic Function | Glucocorticoid | | 一般的な説明 | Triamcinolone acetonide is approximately 8 times morepotent than prednisone in animal inflammation models.Topically applied triamcinolone acetonide is a potent antiinflammatoryagent, about 10 times more sothan triamcinolone. The plasma half-life is approximately 90minutes, although the plasma half-life and biological halflivesfor GCs do not correlate well. The hexacetonide isslowly converted to the acetonide in vivo and is given onlyby intra-articular injection. Only triamcinolone and the diacetateare given orally. The acetonide and diacetate may begiven by intra-articular or intrasynovial injection. In addition,the acetonide may be given by intrabursal or, sometimes,IM or subcutaneous injection. A single IM dose of thediacetate or acetonide may last up to 3 or 4 weeks. Plasmalevels with IM doses of the acetonide are significantly higherthan with triamcinolone itself. The acetonide is also used totreat asthma and allergic rhinitis. | | 一般的な説明 | The three main metabolitesof triamcinolone acetonide (Azmacort, Nasacort) are 6β-hydroxytriamcinolone acetonide, 21-carboxytriamcinoloneacetonide, and 6β-hydroxy-21-carboxytriamcinolone acetonide.All are much less active than the parent compound.The 6β-hydroxyl group and the 21-carboxy group are bothstructural features that greatly reduce GC action. The increasedwater solubility of these metabolites also facilitatesmore rapid excretion. | | 臨床応用 | Triamcinolone acetonide used topically to treat various skin conditions, to relieve the discomfort of mouth sores, and by injection into joints to treat various joint conditions.Triamcinolone acetonide frequently is used by inhalation for the treatment of lung diseases (e.g., asthma). | | 副作用 | The side effects of using Triamcinolone acetonide include:Skin dryness, flaking, crusting, burning, or blistering; Skin irritation; Skin soreness, itching, swelling, scaling, or severe redness; Scaling or redness near mouth; Skin thinning or bruising, especially in skin folds (like between the finger) or on the face (when directed to use it there); New or worsening pimples or acne; Skin burning and itching with tiny red blisters; Skin softening; Itching, pain, or burning sensation in hairy areas, or pus at the root of the hair; Increased hair growth on the legs, arms, back, or forehead; Lightening of skin tone; Red or purple lines on the arms, face, legs, groin, or trunk. | | 安全性プロファイル | Poison by
subcutaneous and intraperitoneal routes.
An experimental teratogen. Other
experimental reproductive effects. Human
mutation data reported. When heated to
decomposition it emits acrid smoke and
toxic fumes of F-. | | Veterinary Drugs and Treatments | The systemic veterinary labeled product (Vetalog? Injection) is labeled
as “indicated for the treatment of inflammation and related
disorders in dogs, cats, and horses. It is also indicated for use in
dogs and cats for the management and treatment of acute arthritis,
allergic and dermatologic disorders.”
Glucocorticoids have been used in an attempt to treat practically
every malady that afflicts man or animal, but there are three broad
uses and dosage ranges for use of these agents. 1) Replacement of
glucocorticoid activity in patients with adrenal insufficiency, 2)
as an antiinflammatory agent, and 3) as an immunosuppressive.
Among some of the uses for glucocorticoids include treatment of: endocrine conditions (e.g., adrenal insufficiency), rheumatic diseases
(e.g., rheumatoid arthritis), collagen diseases (e.g., systemic
lupus), allergic states, respiratory diseases (e.g., asthma), dermatologic
diseases (e.g., pemphigus, allergic dermatoses), hematologic
disorders (e.g., thrombocytopenias, autoimmune hemolytic anemias),
neoplasias, nervous system disorders (increased CSF pressure),
GI diseases (e.g., ulcerative colitis exacerbations), and renal
diseases (e.g., nephrotic syndrome). Some glucocorticoids are used
topically in the eye and skin for various conditions or are injected
intra-articularly or intra-lesionally. The above listing is certainly
not complete. | | 代謝 | Triamcinolone acetonide frequently is used by inhalation for the treatment of lung diseases (e.g., asthma). After
inhalation, triamcinolone acetonide can become systemically available when the inhaled formulation is
swallowed and absorbed unchanged from the GI tract, causing undesirable systemic effects.
Triamcinolone acetonide that is swallowed is metabolized to 6β-hydroxytriamcinolone acetonide,
21-carboxytriamcinolone acetonide, and 21-carboxy-6β-hydroxytriamcinolone acetonide, all of which are more
hydrophilic than their parent drug. Only approximately 1% of the dose was recovered from the urine as
triamcinolone acetonide. Triamcinolone is not a major metabolite of triamcinolone acetonide in humans,
suggesting that acetonide is resistant to hydrolytic cleavage. Triamcinolone acetonide is approximately eight
times more potent than prednisolone. |
|