|
ChemicalBook Optimization Suppliers |
| 名前: |
Energy Chemical |
| 電話番号: |
021-021-58432009 400-005-6266 |
| 電子メール: |
sales8178@energy-chemical.com |
|
| 化学名: | フラボキサート塩酸塩 | | 英語化学名: | Flavoxate hydrochloride | | 别名: | FLAVOMYCINPREMIX;1-piperidineethanol,3-methyl-4-oxo-2-phenyl-4h-1-benzopyran-8-carboxylate,h;2-piperidinoethyl-3-methyl-4-oxo-2-phenyl-4h-1-benzopyran-8-carboxylatehydro;3-methyl-4-oxo-2-phenyl-4h-1-benzopyran-8-carboxylicaci2-piperidinoethyl;bladderon;Urispas;Flavoxate hydrochloride,3-Methyl-4-oxo-2-phenyl-4H-1-benzopyran-8-carboxylic acid 2-(1-piperidinyl)ethyl ester hydrochloride, DW-61, Rec-7-0040;Flavoxate Hydrochloride (200 mg) | | CAS番号: | 3717-88-2 | | 分子式: | C24H26ClNO4 | | 分子量: | 427.92 | | EINECS: | 223-066-4 | | カテゴリ情報: | Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;ACTIVE PHARMACEUTICAL INGREDIENTS;API | | Mol File: | 3717-88-2.mol | 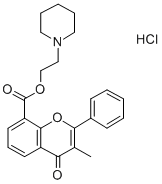 |
| 融点 | 232-234°C | | 貯蔵温度 | Inert atmosphere,Room Temperature | | 溶解性 | H2O: ~6.6 mg/mL | | 外見 | solid | | 色 | white | | InChI | InChI=1S/C24H25NO4.ClH/c1-17-21(26)19-11-8-12-20(23(19)29-22(17)18-9-4-2-5-10-18)24(27)28-16-15-25-13-6-3-7-14-25;/h2,4-5,8-12H,3,6-7,13-16H2,1H3;1H | | InChIKey | XOEVKNFZUQEERE-UHFFFAOYSA-N | | SMILES | C12OC(=C(C)C(=O)C=1C=CC=C2C(=O)OCCN1CCCCC1)C1C=CC=CC=1.Cl | | CAS データベース | 3717-88-2(CAS DataBase Reference) |
| 主な危険性 | Xn | | Rフレーズ | 22-36/37/38 | | Sフレーズ | 26 | | WGK Germany | 3 | | RTECS 番号 | DJ2450000 | | HSコード | 29349990 | | 毒性 | LD50 i.v. in rats: 27.4 mg/kg (Cazzulani) |
| | フラボキサート塩酸塩 Usage And Synthesis |
| 外観 | 白色, 結晶性粉末~粉末 | | 溶解性 | 水に溶ける。 | | 用途 | 薬理研究用。 | | 用途 | 膀胱平滑筋において、収縮抑 制作用を示します。 | | 用途 | 膀胱平滑筋において、収縮抑
制作用を示します。 | | 効能 | 頻尿治療薬, ホスホジエステラーゼ阻害薬 | | 商品名 | ブラダロン (日本新薬) | | 化学的特性 | Flavoxate hydrochloride is Crystalline Solid | | Originator | Urispas,SKF,US,1971 | | 使用 | Smooth muscle relaxant. Used as antispasmodic; in treatment of urinary incontinence. | | 使用 | Antispasmodic;Phosphodiesterase inhibitor | | 定義 | ChEBI: The hydrochloride salt of flavoxate. | | Manufacturing Process | A mixture of 13.3 grams of anhydrous aluminum chloride and 100 ml of
carbon disulfide is added to 19.4 grams of 2-propionyloxybenzoic acid
(prepared from the reaction of propionyl chloride and 2-hydroxybenzoic acid).
After an initial evolution of hydrogen chloride, the solvent is removed by
distillation and the mixture is heated at 150° to 160°C for 4 hours. The cooled
reaction mixture is treated with ice and hydrochloric acid and the product, 2-
hydroxy-3-carboxypropiophenone, is obtained from the oily residue by
distillation in vacuo.
A mixture of 1.9 grams of 2-hydroxy-3-carboxypropiophenone, 5.0 grams of
sodium benzoate and 20.0 grams of benzoic anhydride is heated at 180° to
190°C for 6 hours. A solution of 15.0 grams of potassium hydroxide in 50 ml
of ethanol and 20 ml of water is added and refluxed for 1 hour. The mixture is
evaporated and the residue after addition of water yields 3-methylflavone-8-
carboxylic acid.
To a suspension of 12.0 grams of 3-methylflavone-8-carboxylic acid in 200 ml
of anhydrous benzene is added 10.0 grams of thionyl chloride. The mixture is
refluxed for 2 hours during which the suspended solid goes into solution. The
solvent is completely removed by distillation, the residue extracted with
benzene and the extract evaporated to dryness. The product, 3-
methylflavone-8-carboxylic acid chloride, is recrystallized from ligroin to give
crystals melting at 155° to 156°C.
To 11.0 grams of 3-methylflavone-8-carboxylic acid chloride dissolved in 150
ml of anhydrous benzene is added at room temperature 4.8 grams of
piperidinoethanol and the mixture refluxed for 2 to 3 hours. The separated
solid is filtered, washed with benzene and dried. The product, piperidinoethyl
3-methylflavone-8-carboxylate hydrochloride is obtained as a colorless
crystalline solid, MP 232° to 234°C, (from US Patent 2,921,070). | | brand name | Urispas (Ortho-McNeil). | | Therapeutic Function | Spasmolytic | | 副作用 |
The following adverse reactions have been observed, but there are not enough data to support an estimate of their frequency.
Gastrointestinal: Nausea, vomiting, dry mouth.
CNS: Vertigo, headache, mental confusion, especially in the elderly, drowsiness, nervousness.
Hematologic: Leukopenia (one case which was reversible upon discontinuation of the drug).
Cardiovascular: Tachycardia and palpitation.
Allergic: Urticaria and other dermatoses, eosinophilia and hyperpyrexia.
Ophthalmic: Increased ocular tension, blurred vision, disturbance in eye accommodation.
Renal: Dysuria.
| | Veterinary Drugs and Treatments | Flovoxate may be considered for treating dogs with detrusor hyperspasticity
(hyperactive bladder, urge incontinence). | | 薬理学 | Flavoxate hydrochloride counteracts smooth muscle spasm of the urinary tract and exerts its effect directly on the muscle. In a single study of 11 normal male subjects, the time to onset of action was 55 minutes. The peak effect was observed at 112 minutes. 57% of the flavoxate hydrochloride was excreted in the urine within 24 hours. |
|