| Company Name: |
Beijing Comparison Pharmaceutical Technology Co., Ltd
|
| Tel: |
010-52878169 15701683040 |
| Email: |
sales@bjcomparison.com |
| Products Intro: |
Product Name:(3RS)-3-[(1H-imidazol-1-yl)methyl]-9-methyl-1,2,3,9- tetrahydro-4H-carbazol-4-one
CAS:99614-04-7
Purity:≥98% Package:100mg
|
| Company Name: |
Riedel-de Haen AG
|
| Tel: |
800 558-9160 |
| Email: |
|
| Products Intro: |
Product Name:(3RS)-3-(1H-Imidazol-1-ylmethyl)-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one monohydrochloride
CAS:99614-04-7
|
|
| | Ondansetron impurity G (PhEur) Basic information |
| Product Name: | Ondansetron impurity G (PhEur) | | Synonyms: | (3RS)-3-(1H-Imidazol-1-ylmethyl)-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one monohydrochloride;Ondansetron impurity G (PhEur);Ondansetron Impurity 03 | | CAS: | 99614-04-7 | | MF: | C17H18ClN3O | | MW: | 315.8 | | EINECS: | | | Product Categories: | | | Mol File: | 99614-04-7.mol | 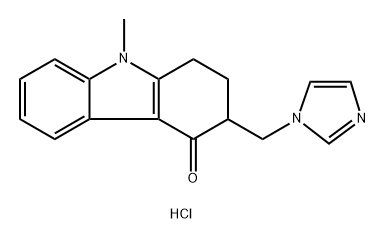 |
| | Ondansetron impurity G (PhEur) Chemical Properties |
| Hazard Codes | Xi | | Risk Statements | 37/38-41 | | Safety Statements | 26-39 | | WGK Germany | 3 |
| | Ondansetron impurity G (PhEur) Usage And Synthesis |
| | Ondansetron impurity G (PhEur) Preparation Products And Raw materials |
|