|
ChemicalBook Optimization Suppliers |
|
| 融点 | −40 °C(lit.) | | 沸点 | 57 °C(lit.) | | 比重(密度) | 0.857 g/mL at 25 °C | | 蒸気密度 | 3.7 (vs air) | | 蒸気圧 | 100 mm Hg ( 25 °C) | | 屈折率 | n20/D 1.500 | | 闪点 | 104 °F | | 貯蔵温度 | Store below +30°C. | | 溶解性 | Miscible with ether, benzene, diethylether and perchloroethylene. | | 外見 | Liquid | | 比重 | 0.8536 (27℃) | | 色 | Clear colorless | | 爆発限界(explosive limit) | 1.5-46%(V) | | 水溶解度 | REACTS | | Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents | | Sensitive | Moisture Sensitive | | BRN | 1209232 | | 安定性: | Stable. Highly flammable - note low flash point. Reacts violently with water. Incompatible with water, moisture, strong oxidizing agents, strong acids, strong bases, aldehydes, alcohols, amines, esters, ketones. | | InChIKey | IJOOHPMOJXWVHK-UHFFFAOYSA-N | | CAS データベース | 75-77-4(CAS DataBase Reference) | | NISTの化学物質情報 | Silane, chlorotrimethyl-(75-77-4) | | EPAの化学物質情報 | Trimethylchlorosilane (75-77-4) |
| | クロロトリメチルシラン Usage And Synthesis |
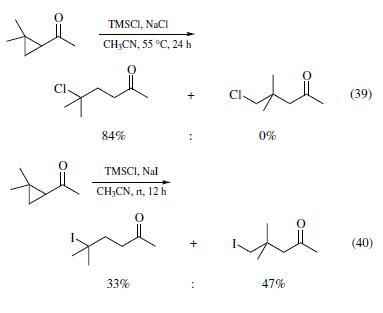
| 外観 | 無色~ほとんど無色, 澄明の液体 | | 溶解性 | ジエチルエーテルに極めて溶けやすく、水及びエタノールとは反応する。 | | 解説 | クロロトリメチルシラン,金属ケイ素と塩化メチルを銅触媒を用いて高温で反応させるRochowの直接法によって合成される.刺激臭のある無色の液体.融点-40 ℃,沸点57.9 ℃.0.854.1.389.水とはげしく反応し,塩化水素を副生してヘキサメチルジシロキサンを生成する.種々の求核試剤と反応し,合成化学的に有用なシリルエーテル,有機ケイ素化合物などの合成原料となる.腐食性および刺激性がある. | | 用途 | ガスクロマトグラフ分析における試料の前処理剤(トリメチルシリル化剤)。有機合成:和光試薬時報Vol.64 No.2,p.26(1996)、Vol.61 No.3,p.22(1993)。 | | 用途 | 主たる用途は????????化剤として合成反応に使用される。また、シリコーンオイル、シリコーン系撥水剤等のメチル系シロキサンポリマーの重合鎖末端停止剤である。 | | 説明 | Chlorotrimethylsilane is a valuable reagent for the protection of the hydroxyl function in organic synthesis. It should be distilled from calciumhydride (or tributylamine) under nitrogen before use,and stored and weighed under nitrogen. Use of the reagent without purification has been reported to lead to explosions. Chlorotrimethylsilane may contain dichlorodimethylsilane as an impurity. This may be removed before distillation by very cautious treatment with a small amount of water,which hydrolyses the dichloro compound more rapidly. Excess chlorotrimethylsilane in a reaction mixture may be destroyed by the very careful addition of aqueous sodium hydrogencarbonate solution. | | 化学的特性 | Trimethylchlorosilane is a colorless fuming liquid with a pungent odor. Readily hydrolyzed with liberation of hydrogen chloride; soluble in benzene, ether and perchloroethylene. Chlorotrimethylsilane is a chloro-organosilane compound mainly used for silylation reactions. | | 反応性 | Related to the epoxide ring opening, TMSCl also mediates some cyclopropane ring opening reactions. For example, treatment of 1-aceto-2,2-dimethylcyclopropane with TMSCl and sodium chloride in acetonitrile at 55°C for 24 h generated 5-chloro-5-methyl-2-hexanone in 84% yield (eq 39).When sodium iodide was employed to replace sodium chloride, iodotrimethylsilane generated in situ, and the reaction completed under more facile conditions (rt and 12 h). Interestingly, the dominant product is 5-iodo-4,4-dimethyl-2-pentanone (eq 40), arising from iodide attacking at the less hindered secondary methylene carbon, instead of the quaternary dimethylmethylene carbon.
 | | 主な応用 | Trimethylsilyl chloride(TMCS) is the most extensively used derivatization reagent for gas chromatography.The reactions are generally performed under anhydrous conditions although reactions can be performed on samples obtained from water-containing samples.There are considerable opportunities for artifacts stemming from a variety of reasons including off-target reactions with aldehydes and ketones.The rate of reaction of TMCS with nucleophilic targets is comparatively slow compared to other alkylsilyl chlorides and requires the presence of a base catalyst such as pyridine.TMCS is included as a catalyst with other trialkylsilyl reagent such N,O-bis- (trimethylsilyl)trifluroacetamide (BSA) or N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA).The order of functional group reactivity is alcohols>phenols>carboxylic acids>amines>amides. | | 使用 | Chlorotrimethylsilane is a typical Silane Blocking Agent, which can protect or deprotect functional groups selectively. It have been used in the preparation of volatile derivatives of a wide range of compounds for GC analysis, and used for silylation and as a protection group in the process of various organic synthesis. It is used in the production of trimethylsilyl halides, pseudohalides and various organic silicon compounds. It is also used to produce hexamethyldisilane by reduction. | | 定義 | ChEBI: Chlorotrimethylsilane is a silyl chloride consisting of a central silicon atom covalently bound to one chloro and three methyl groups. Chlorotrimethylsilane is a derivatisation agent used in gas chromatography/mass spectrometry applications. It has a role as a chromatographic reagent. | | 一般的な説明 | Trimethylchlorosilane appears as a colorless fuming liquid with a pungent odor. Boiling point 135° F, Flash point -18°F. Density 0.854g/cm3. The vapor and liquid may cause burns. Vapors are heavier than air. | | 反応プロフィール | Chlorotrimethylsilane reacts vigorously and exothermically with water to produce hydrogen chloride. | | 健康ハザード | Similar to other silanes. Toxicity is rated high for inhalation, ingestion and local irritation. May cause death or permanent injury after a very short exposure to small quantities. | | 火災危険 | Violent reaction with water. Toxic and irritating hydrogen chloride and phosgene may be formed in fires. Difficult to extinguish, re-ignition may occur. Flashback along vapor trail may occur. Containers may explode in fire. Vapor may explode if ignited in enclosed area. When heated to decomposition or on contact with acids or acid fumes, chloride fumes are emitted. Reacts with surface moisture, releasing hydrogen chloride, which will corrode common metals and form flammable hydrogen gas. Avoid contact with water; Chlorotrimethylsilane readily hydrolyzes, liberating hydrochloric acid. Hazardous polymerization may not occur. | | 安全性プロファイル | Poison by ingestion and
skin contact. Moderately toxic by inhalation
and intraperitoneal routes. A corrosive
irritant to skin, eyes, and mucous
membranes. Questionable carcinogen with
experimental neoplastigenic data. Mutation
data reported. A flammable liquid and very
dangerous fire hazard when exposed to heat
or flame. Violent reaction with water or
hexafluoroisopropylideneamino lithium, A
preparative hazard. To fight fire, use foam,
alcohol foam, fog. When heated to
decomposition it emits toxic fumes of Cl-.
An intermediate in the production of
silicones. See also CHLOROSILASES. | | 職業ばく露 | Trimethylchlorosilane is used as an
intermediate to make silicone products, including lubricants | | 輸送方法 | UN1298 Trimethylchlorosilane, Hazard Class: 3;
Labels: 3-Flammable liquid, 8-Corrosive material. | | 純化方法 | Likely impurities are other chlorinated methylsilanes and tetrachlorosilane (b 57.6o), some of which can form azeotropes. To avoid the latter, very efficient fractional distillation is required. It has been fractionated through a 12 plate glass helices-packed column with only the heart-cut material being used. It has also been fractionated through a 90cm, 19mm diameter Stedman column (p 11). Purify it by redistilling from CaH2 before use. [Sauer et al. J Am Chem Soc 70, 4254 1948, Sauer & Hadsell J Am Chem Soc 70 4258 1948, Langer et al. J Org Chem 23 50 1958, Beilstein 4 IV 4007.] FLAMMABLE and CORROSIVE. | | 不和合性 | Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides. Chlorosilanes react
vigorously with bases and both organic and inorganic acids
generating toxic and/or flammable gases. Chlorosilanes
react with water, moist air, or steam to produce heat and
toxic, corrosive fumes of hydrogen chloride. They may also
produce flammable gaseous hydrogen. Attacks metals in
the presence of moisture. Vigorous reaction with aluminum
powder. | | 廃棄物の処理 | Do not discharge into drains
or sewers. Use a licensed disposal contractor to an
approved landfill. Must be disposed properly by following
package label directions or by contacting your local or
federal environmental control agency, or by contacting
your regional EPA office for guidance on
acceptable disposal practices. The most favorable course
of action is to use an alternative chemical product with
less inherent propensity for occupational exposure or environmental
contamination. Recycle any unused portion of
the material for its approved use or return it to the manufacturer
or supplier. Ultimate disposal of the chemical
must consider: the material’s impact on air quality; potential
migration in soil or water; effects on animal, aquatic,
and plant life; and conformance with environmental and
public health regulations. |
|