|
ChemicalBook Optimization Suppliers |
|
| 化学名: | ゲムシタビン | | 英語化学名: | Gemcitabine | | 别名: | 2'-Deoxy-2',2'-difluoro-D-cytidine;2''-DEOXY-2'',2''-DIFLUOROCYTIDINE(GEMCITABINE);2',2'-Difluorodeoxycytidine;Cytidine, 2'-deoxy-2',2'-difluoro-;NSC 613327;gencitabine base;2'-Deoxy-2',2'-difluro cytidine Hydrochloride;GEMCITABINE (2''-DEOXY-2'', 2''-DIFLUOROCYTIDINE) | | CAS番号: | 95058-81-4 | | 分子式: | C9H11F2N3O4 | | 分子量: | 263.2 | | EINECS: | 619-100-6 | | カテゴリ情報: | Inhibitors;Antineoplastic;API;Antineoplastic drug, difluorine nucleoside analog | | Mol File: | 95058-81-4.mol | 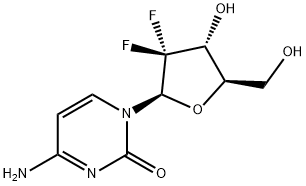 |
| 融点 | 168,64 C | | 比旋光度 | 365 +425.36°; D +71.51° | | 沸点 | 482.7±55.0 °C(Predicted) | | 比重(密度) | 1.84±0.1 g/cm3(Predicted) | | 貯蔵温度 | Keep in dark place,Sealed in dry,Store in freezer, under -20°C | | 溶解性 | Methanol (Slightly), Water (Slightly, Heated) | | 酸解離定数(Pka) | 11.65±0.70(Predicted) | | 外見 | Solid | | 色 | White to Off-White | | CAS データベース | 95058-81-4(CAS DataBase Reference) |
| | ゲムシタビン Usage And Synthesis |
| 外観 | 白色~うすい黄色、結晶性粉末~粉末 | | 用途 | ゲムシタビン(Gemcitabine、略号:GEM)とは、抗癌剤として用いられる含フッ素ヌクレオシドの一種である。
| | 効能 | 抗悪性腫瘍薬, 代謝拮抗薬 | | 使用上の注意 | アルゴン封入 | | 説明 | Gemcitabine is an anticancer nucleoside analog that inhibits the growth of HL-60 promyelocytic leukemia cells with an LC50 value of 40 nM. It inhibits the growth of MX-1 mammary, CX-1, HC-1, GC3, and VRC5 colon, LX-1, Calu-6, and NCI-H460 lung, and HS766T, PaCa-2, PANC-1, and BxPC-3 pancreatic cancer tumors in mouse xenograft models (45-93% inhibition). Gemcitabine is a prodrug that is metabolized to a diphosphate and triphosphate form in cells. The triphosphate form is incorporated into DNA which induces masked chain termination and cell death. By specifically inhibiting growth arrest and DNA damage inducible protein 45 a (Gadd45a), a key mediator of active DNA demethylation, gemcitabine, at concentrations ranging from 34 to 134 nM, inhibits repair-mediated DNA demethylation in a methylation-sensitive reporter assay. Gemcitabine also has broad antiretroviral activity, decreasing MuLV cell infectivity, a murine AIDS model, in cell culture (EC50 = ~1.5 nM) and inhibits the progression of murine AIDS in vivo at a dose of 1-2 mg/kg per day. | | Originator | Gemzar,Lilly Co. | | 使用 | Gemcitabine is used for breast cancer treatment. First-line treatment for locally advanced pancreatic cancer. | | 使用 | Gemcitabine(Gemzar) belongs to the group of medicines called antimetabolites. It is used alone or in combination with other medicines to treat cancer of the breast, ovary, pancreas, and lung. Gemcitabine interferes with the growth of cancer cells, which a | | 適応症 | Gemcitabine (Gemzar), an antimetabolite, undergoes
metabolic activation to difluorodeoxycytidine triphosphate,
which interferes with DNA synthesis and repair.
It is the single most active agent for the treatment of
metastatic pancreatic cancer, and it is used as a first-line
treatment for both pancreatic and small cell lung cancer.
It is administered by intravenous infusion. The
dose-limiting toxicity is bone marrow suppression. | | 定義 | ChEBI: Gemcitabine is a 2'-deoxycytidine having geminal fluoro substituents in the 2'-position. An inhibitor of ribonucleotide reductase, gemcitabine is used in the treatment of various carcinomas, particularly non-small cell lung cancer, pancreatic cancer, bladder cancer and breast cancer. It has a role as a photosensitizing agent, a DNA synthesis inhibitor, a prodrug, an EC 1.17.4.1 (ribonucleoside-diphosphate reductase) inhibitor, an environmental contaminant, a xenobiotic, a radiosensitizing agent, an antineoplastic agent, an antimetabolite, an antiviral drug and an immunosuppressive agent. It is an organofluorine compound and a pyrimidine 2'-deoxyribonucleoside. | | Manufacturing Process | Benzyl 4,6-O-benzylidene-2-O-benzyl-3-oxo-α-D-gluco-pyranoside was
obtained by 4 steps from glucose.
0.53 ml (4.0 mmol) of DAST (fluorinaiting agent) was added to asolution of
300 mg (0.67 mmol) of benzyl 4,6-O-benzylidene-2-O-benzyl-3-oxo-α-D�gluco-pyranoside in anhydrous dichloromethane (4 ml). The solution was then
stirred at room temperature for 2 h, and the excess of DAST was neutralized
by careful addition of saturated aqueous NaHCO3. The resulting mixture was
extracted with CH2Cl2, and organic phase was dried and evaporated. The
residue was purified by CC (Hexane/Ethyl acetate 7:1) to afford benzyl 4,6-O�benzylidene-2-O-benzyl-3-deoxy-3,3-difluoro-α-D-gluco-pyranoside (189 mg,
60%), melting point 118°-119°C.
Benzyl 4,6-O-benzylidene-2-O-benzyl-3-deoxy-3,3-difluoro-α-D-gluco�pyranoside (77 mg, 0.16 mmol) was dissolved in a 0.1 N solution of HCl in
ethanol and stirred at room temperature for 40 h. The solution was then
neutralized with solid NaHCO3, filtered and evaporated to give an oily product
that was dissolved in 2 ml of CH2Cl2 and 0.5 ml of pyridine. After cooling to
0°C, 0.40 ml (1.6 mmol) of benzoyl chloride was added and the solution was
stirred for 1 h and poured into ice and water (200 ml) containing NaHCO3,
extracted several times with CH2Cl2, dried and evaporated to give 86 mg
(0.14 mmol, 90%) of benzyl 4,6-di-O-benzoyl-2-O-benzyl-3-deoxy-3,3-
difluoro-α-D-gluco-pyranoside.
Benzyl 4,6-di-O-benzoyl-2-O-benzyl-3-deoxy-3,3-difluoro-α-D-gluco�pyranoside (220 mg, 0.44 mmol) was dissolved in methanol in the presence
of 200 mg of palladium on activated charcoal (10% Pd content). The
suspension was stirred at room temperature under hydrogen pressure (10
bar) for 16 h. The suspension was then filtered through a thin silica gel pad,
and evaporated. The residue was purified by CC to give 105 mg (59%) of 4,6-
di-O-benzoyl-3-deoxy-3,3-difluoro-α/β-D-gluco-pyranoside as an inseparable
anomeric mixture (ratio α/β = 5:1).
To a solution of 46 mg (0.11 mmol) of 4,6-di-O-benzoyl-3-deoxy-3,3-difluoro-
α/β-D-gluco-pyranoside in water-dioxane 1:2 (2 ml) was added 120 mg (0.56
mmol) of sodium periodate. This resulting solution was stirred at room
temperature for 20 h. Then, more sodium periodate (55 mg, 0.26 mmol) was
added and stirring was continued for 6 h. After that, the solvents were
evaporated and the solid was repeatedly extracted with ethyl acetate (total
volume 70 ml). The solvent was then evaporated to give a solid that was
treated for 15 min with a diluted (0.1%) methanolic solution of ammonia. THE
solution was evaporated and the crude purified by preparative TLC
(hexane/ethyl acetate 2:1) to yield 18 mg (0.04 mmol, 43%) of α-3,5-di-O�benzoyl-2-deoxy-2,2-difluoro-D-ribose. | | Therapeutic Function | Antineoplastic, Antiviral | | 一般的な説明 | The drug is available as the hydrochloride salt in 200- and1,000-mg lyophilized single-dose vials for IV use.Gemcitabine is used to treat bladder cancer, breast cancer,pancreatic cancer, and NSCLC. Gemcitabine is a potent radiosensitizer,and it increases the cytotoxicity of cisplatin.The mechanism of action of this fluorine-substituted deoxycytidineanalog involves inhibition of DNA synthesis andfunction via DNA chain termination. The triphosphatemetabolite is incorporated into DNA inhibiting severalDNA polymerases and incorporated into RNA inhibitingproper function of mRNA. Resistance can occur because ofdecreased expression of the activation enzyme deoxycytidinekinase or decreased drug transport as well as increasedexpression of catabolic enzymes. Drug oral bioavailabilityis low because of deamination within the GI tract, and thedrug does not cross the blood-brain barrier. Metabolism bydeamination to 2', 2'-difluorouridine (dFdU) is extensive.Drug toxicity includes myelosuppression, fever, malaise,chills, headache, myalgias, nausea, and vomiting. | | 危険性 | Human systemic effects | | 作用機序 | Gemcitabine shows
good activity against human leukemic cell lines,
a number of murine solid tumors, and human
tumor xenografts. Gemcitabine was
significantly more cytotoxic than cytarabine in
Chinese hamster ovary cells. The major cellular
metabolite is the 5'-triphosphate of gemcitabine.
The cytotoxicity was competitively reversed by
deoxycytidine, suggesting that the biological
activity required phosphorylation by deoxycytidine kinase. Tumor-bearing mice were treated with either gemcitabine or cytarabine (20 mg/kg). DNA
synthesis reached 1 % of control levels upon
administration of gemcitabine. The greater
accumulation of gemcitabine-5'-triphosphate
compared with cytarabine-5'-triphosphate may
cause greater cytotoxicity and therapeutic activity. Further gemcitabine may enhance its own cytotoxic effects by self-potentiation mechanisms that act on, e. g., deoxycytidine monophosphate
deaminase, deoxycytidine kinase or on DNA synthesis. | | 臨床応用 | Antineoplastic agent:
Palliative treatment, or first-line treatment with
cisplatin, of locally advanced or metastatic non-small
cell lung cancer
Pancreatic, ovarian and breast cancer
Bladder cancer in combination with cisplatin | | 薬物相互作用 | Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis. | | 代謝 | After intravenous doses gemcitabine is rapidly cleared
from the blood and metabolised by cytidine deaminase
in the liver, kidney, blood, and other tissues. Clearance is
about 25% lower in women than in men.
Almost all (99%) of the dose is excreted in urine as
2′-deoxy-2′,2′-difluorouridine (dFdU), only about
1% being found in the faeces. Intracellular metabolism
produces mono-, di-, and triphosphate metabolites, the
latter two active. The active intracellular metabolites have
not been detected in plasma or urine. | | 参考文献 | [1] karnitz lm, flatten ks, wagner jm, et al. gemcitabine-induced activation of checkpoint signaling pathways that affect tumor cell survival. mol pharmacol, 2005, 68 (6): 1636-1644.
[2] ando t, ichikawa j, okamoto a, et al. gemcitabine inhibits viability, growth, and metastasis of osteosarcoma cell lines. j orthop res, 2005, 23 (4): 964-969.
[3] clouser cl, holtz cm, mullett m, et al. analysis of the ex vivo and in vivo antiretroviral activity of gemcitabine. plos one, 2011, 6 (1): e15840. |
|