|
ChemicalBook Optimization Suppliers |
|
| 化学名: | ビタミン K3 | | 英語化学名: | Menadione | | 别名: | Vitamin K3 (MSB/MNB);Menadione,2-Methyl-1,4-Napthoquinone;Sodium bisulfite of 2-methyl-1,4-naphthoquinone;2-Methyl-1,4-naphthoquinone,98%;2-Methyl-1,4-naphthoquinone, Menadione, VitaminK3;2-Methyl-1,4-naphthoquinone, VitaminK3;Menaquinone-7 (Vitamin K2);MENADIONE CRYSTALLINE | | CAS番号: | 58-27-5 | | 分子式: | C11H8O2 | | 分子量: | 172.18 | | EINECS: | 200-372-6 | | カテゴリ情報: | Biochemistry;Vitamins;Nutritional fortification substances;Vitamins and derivatives;Vitamin Ingredients;Miscellaneous Compounds;feeding additive raw materials;Inhibitors;RUMENSIN;Isolabel;Other APIs;Aromatics;Intermediates & Fine Chemicals;Pharmaceuticals;58-27-5 | | Mol File: | 58-27-5.mol | 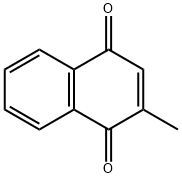 |
| 融点 | 105-107 °C(lit.) | | 沸点 | 262.49°C (rough estimate) | | 比重(密度) | 1.1153 (rough estimate) | | 屈折率 | 1.5500 (estimate) | | 貯蔵温度 | room temp | | 溶解性 | oil: soluble | | 外見 | crystalline | | 色 | yellow | | 臭い (Odor) | Slight odor | | 水溶解度 | INSOLUBLE | | Sensitive | Light Sensitive | | Merck | 14,5831 | | BRN | 1908453 | | 安定性: | Stable. May be light sensitive. Incompatible with strong oxidizing agents. | | InChIKey | MJVAVZPDRWSRRC-UHFFFAOYSA-N | | LogP | 2.200 | | CAS データベース | 58-27-5(CAS DataBase Reference) | | NISTの化学物質情報 | Menadione(58-27-5) | | EPAの化学物質情報 | Menadione (58-27-5) |
| | ビタミン K3 Usage And Synthesis |
| 外観 | うすい黄色~黄色~緑色粉末~結晶 | | 定義 | 本品は、次の化学式で表される多環式芳香族化合物である。 | | 性質 | ビタミン K3:C11H8O2(172.18).メナジオン(menadion)ともいう.天然には存在しない合成ビタミン.2-メチルナフタレンのクロム酸酸化により合成される.黄色の結晶.かすかな刺激臭をもち,粘膜,呼吸器,皮膚などを刺激する.融点105~107 ℃.毒性の面で医薬としての利用は禁止されているが,配合飼料や動物医薬用として相当量合成されている. | | 用途 | ビタミンKの前駆体ではあるが、先進国ではサプリメントとしては用いられない。
低プロトロンビン血症の治療にも用いられる。 | | 効能 | 止血薬, ビタミンK補充薬 | | 説明 | Vitamin K is a general term referring to a group of naphthoquinone derivatives required in the diet for blood clotting. Menadione is a fat-soluble vitamin that is essential for blood clotting. It is destroyed by irradiation during processing but has no appreciable loss during storage. It occurs in spinach, cabbage, liver, and wheat bran. | | 化学的特性 | Bright-yellow crystals with a very faint acrid odour. Insoluble in water; soluble in benzene (1 g/10 mL), ethanol (1 g/60 mL), and vegetable oils (1g/50 mL); moderately soluble in carbon tetrachloride and chloroform. Stable in air; decomposed by sunlight; destroyed by alkalis and reducing agents. | | 物理的性質 | Appearance: phylloquinone is a yellow oil at room temperature, but the other vitamers K are yellow crystals. Solubility: the vitamers K and MK and most forms of menadione are insoluble in water, slightly soluble in ethanol, and readily soluble in ether, chloroform, fats, and oils. Stability: the vitamers K are sensitive to light and alkali, but are relatively stable to heat and oxidizing environments.
Menadione, the formal parent compound of the menaquinone series does not
occur naturally but is a common synthetic form called menadione (2-methyl-1,4-
naphthoquinone). This compound forms a water-soluble sodium bisulfite addition
product, menadione sodium bisulfite, whose practical utility is limited by its instability in complex matrices such as feeds. However, in the presence of excess sodium
bisulfite, it crystallizes as a complex with an additional mole of sodium bisulfite
(i.e., menadione sodium bisulfite complex), which has greater stability, therefore, is
used widely as a supplement to poultry feeds. A third water-soluble compound is
menadione pyridinol bisulfite (MPB). | | Originator | Kappaxin,Sterling Winthrop | | 使用 | Menadione is precursor to verious types of Vitamin K. It is of industrial importance as an intermediate in the synthesis of phylloquinone, and salts of its bisulfite adduct are used as stabilized forms in the animal feed industry. Commercially significant forms are menadione sodium bisulfite and menadione dimethyl pyrimidinol. Used as a micronutrient for livestock and pet foods. | | 主な応用 | The primary known function of vitamin K is to assist in the normal clotting of blood, but it may also play a role in normal bone calcification. It is being incorporated into cosmetic preparations, particularly those used for treating dark circles. It could also be used in acne products, and there are studies underway on its efficacy for the treatment of spider veins. | | 製造方法 | Menadione can be prepared by oxidizing 2-methylnaphthalene with chromic acid or hydrogen peroxide. | | 定義 | ChEBI: Menadione is a member of the class of 1,4-naphthoquinones that is 1,4-naphthoquinone which is substituted at position 2 by a methyl group. It is used as a nutritional supplement and for the treatment of hypoprothrombinemia. It has a role as a nutraceutical, a human urinary metabolite, an angiogenesis inhibitor, an EC 3.4.22.69 (SARS coronavirus main proteinase) inhibitor and an antineoplastic agent. It is a member of 1,4-naphthoquinones and a vitamin K. | | 適応症 | Vitamin K activity is associated with several quinones,
including phylloquinone (vitamin K1), menadione (vitamin
K3), and a variety of menaquinones (vitamin K2).
These quinones promote the synthesis of proteins that
are involved in the coagulation of blood.These proteins
include prothrombin, factor VII (proconvertin), factor
IX (plasma thromboplastin). The vitamin K quinones are obtained
from three major sources.Vitamin K is present in various
plants, especially green vegetables. The menaquinones
that possess vitamin K2 activity are synthesized
by bacteria, particularly gram-positive organisms;
the bacteria in the gut of animals produce useful quantities
of this vitamin.Vitamin K3 is a chemically synthesized
quinone that possesses the same activity as vitamin
K1. | | brand name | Kappaxin (Sterling Winthrop); Kayquinone. | | Therapeutic Function | Prothrombogenic vitamin | | 一般的な説明 | Menadione is a prothrombogenic compound and belongs to the Vitamin K class of compounds, which are necessary for the biosynthesis of prothrombin and other blood clotting factors. It is used as a model quinone in cell culture and in vivo investigations. It is obtained as a catabolic product of phylloquinone and circulating precursor of tissue menaquinone-4 in rats. | | 危険性 | Irritant to skin and mucous membranes,
especially the alcoholic solution. | | Biochem/physiol Actions | Menadione is an oxidative stress inducer. | | 薬理学 | The typical role of vitamin K is to maintain normal blood coagulation function. Its role is related to the metabolic processes. | | 臨床応用 | Vitamin K deficiency results in increased bleeding
time. This hypoprothrombinemia may lead to hemorrhage
from the gastrointestinal tract, urinary tract, and
nasal mucosa. In normal, healthy adults, deficiency is
rare. The two groups at greatest risk are newborn infants
and patients receiving anticoagulant therapy; hypoprothrombinemia
preexists in these two groups. Any
disease that causes the malabsorption of fats may lead
to deficiency. Inhibition of the growth of intestinal bacteria
from extended antibiotic therapy will result in decreased
vitamin K synthesis and possible deficiency. | | 副作用 | Toxicity of vitamin K has not been well defined.
Jaundice may occur in a newborn if large dosages of vitamin
K are given to the mother before birth. Although
kernicterus may result, this can be prevented by using
vitamin K. | | 安全性プロファイル | Poison by ingestion,
intraperitoneal, and subcutaneous routes.
Experimental teratogenic effects.
Questionable carcinogen with experimental
tumorigenic data. Human mutation data
reported. When heated to decomposition it
emits acrid smoke and irritating fumes. | | 純化方法 | Recrystallise it from 95% EtOH, or MeOH after filtration. It forms bright yellow crystals which are decomposed by light. Its solubility in EtOH is 1.7% and in *C6H6 it is 10%. It IRRITATES mucous membranes and skin. [Fieser J Biol Chem 133 391 1940, Beilstein 7 IV 2430.] |
|