|
ChemicalBook Optimization Suppliers |
|
| 化学名: | o-クレゾール | | 英語化学名: | o-Cresol | | 别名: | O-CRESOL;O-CRESYLIC ACID;O-METHYLPHENOL;FEMA 3480;2-methyl-pheno;1-HYDROXY-2-METHYLBENZENE;o-Cresylic alcohol;Natural O-Cresol | | CAS番号: | 95-48-7 | | 分子式: | C7H8O | | 分子量: | 108.14 | | EINECS: | 202-423-8 | | カテゴリ情報: | alcohol | | Mol File: | 95-48-7.mol | 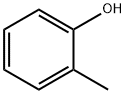 |
| 融点 | 29-31 °C (lit.) | | 沸点 | 191 °C (lit.) | | 比重(密度) | 1.048 g/mL at 25 °C | | 蒸気密度 | 3.72 (vs air) | | 蒸気圧 | 0.3 mm Hg ( 20 °C) | | FEMA | 3480 | O-CRESOL | | 屈折率 | 1.5361 | | 闪点 | 178 °F | | 貯蔵温度 | under inert gas | | 溶解性 | 20g/l | | 外見 | Liquid or Low Melting Solid | | 酸解離定数(Pka) | 10.2(at 25℃) | | 色 | white to brown | | 臭い (Odor) | at 0.01 % in dipropylene glycol. musty phenolic plastic medicinal herbal leathery | | PH | 4.8 (20g/l, H2O, 20℃) | | 爆発限界(explosive limit) | 1.47%, 148°F | | 臭気閾値(Odor Threshold) | 0.00028ppm | | においのタイプ | phenolic | | 水溶解度 | 20 g/L (20 ºC) | | Merck | 14,2579 | | JECFA Number | 691 | | BRN | 506917 | | Henry's Law Constant | 0.34 at 5.25 °C, 0.61 at 10.00 °C, 1.57 at 20.00 °C, 2.33 at 25.00 °C (dynamic equilibrium system-
GC, Feigenbrugel et al., 2004a) | | 暴露限界値 | NIOSH REL: TWA 2.3 ppm (10 mg/m3), IDLH 250 ppm; OSHA PEL: TWA 5
ppm (22 mg/m3); ACGIH TLV: TWA for all isomers 5 ppm (adopted). | | Dielectric constant | 5.8(-5℃) | | 安定性: | Stable, but light and air sensitive. Combustible. Incompatible with oxidizing agents, bases. | | InChIKey | QWVGKYWNOKOFNN-UHFFFAOYSA-N | | LogP | 1.95 at 20℃ | | CAS データベース | 95-48-7(CAS DataBase Reference) | | NISTの化学物質情報 | Phenol, 2-methyl-(95-48-7) | | EPAの化学物質情報 | o-Cresol (95-48-7) |
| | o-クレゾール Usage And Synthesis |
| 外観 | 白色/無色~うすい黄色粉末~塊~透明液体 | | 溶解性 | 水に可溶 (2.0g/100ml水, 20℃), エタノール, エーテルに易溶。エタノール(99.5)及びジエチルエーテルに極めて溶けやすく、水にやや溶けにくく、水酸化ナトリウム溶液に溶ける。 | | 用途 | 防腐剤、消毒剤 | | 用途 | 有機合成原料、溶剤。 | | 用途 | 水質試験用 | | 使用上の注意 | 不活性ガス封入 | | 化学的特性 | Cresol is a mixture of the three isomeric cresols, o-, m-, and p-cresol. Cresols are slightly soluble in water. m-Isomer: Colorless or yellow liquid with characteristic odor. | | 化学的特性 | colourless to light yellow liquid | | 化学的特性 | o-Cresol has a musty, phenolic aftertaste. | | 物理的性質 | Colorless solid or liquid with a phenolic odor; darkens on exposure to air. An odor threshold
concentration of 0.28 ppbv was reported by Nagata and Takeuchi (1990). | | 天然物の起源 | Reported in Acacial farnesiana, ylang-ylang oil (probably as p-cresyl acetate), jasmine absolute, orange oil
from leaves, the essence from flowers of Lilium candidum, anise seed oil, the essence of Artemisia santolinoflia, and some sea algae.
Also reported found in asparagus, peppermint oil, cheddar cheese, provolone cheese, butter, milk, lean fish, boiled egg, smoked pork,
rum, Scotch whiskey, red wine, white wine, coffee and mango.Reported found in cinnamon, coffee, Oriental tobacco, rum, sherry, tea, tomato and whiskey. | | 使用 | Disinfectant; phenolic resins; tricresyl phosphate; ore flotation; textile scouring agent;
organic intermediate; manufacturing salicylaldehyde, coumarin, and herbicides; surfactant;
synthetic food flavors (para isomer only); food antioxidant; dye, perfume, plastics, and resins
manufacturing. | | 使用 | Antiseptics; disinfectants; solvent;
insecticides; resins; flame-retardant plasticizers | | 使用 | o-Cresol is used as a disinfectant and solvent.
Lysol disinfectant is a 50% (v/v) mixed-cresol isomer in
a soap emulsion formed on mixing with water. Besides
disinfection products at solutions of 1–5%, the
cresols are used as degreasing compounds, paintbrush cleaners,
and additives in lubricating oils. Cresols were
previously widely used for disinfection of poultry houses, but
this use was discontinued because of their toxicity; they
cause respiratory problems and abdominal edema in young
chicks. o-Cresol has been used in synthetic resins,
explosives, petroleum, photographic, paint, and agricultural
industries. | | 定義 | ChEBI: A cresol that is phenol substituted by a methyl group at position 2. It is a minor urinary metabolite of toluene. | | 調製方法 | The cresols (cresylic acids) are methyl phenols and generally
appear as a mixture of isomers. o-Cresol is a 2-methyl
derivative of phenol and is prepared from o-toluic acid or obtained from coal tar or petroleum. Crude
cresol is obtained by distilling “gray phenic acid” at a
temperature of ≈180–201°C. o-Cresol may be separated
from the crude or purified mixture by repeated fractional
distillation in vacuo. It can also be prepared synthetically
by diazotization of the specific toluidine or by fusion
of the corresponding toluenesulfonic acid with sodium
hydroxide. | | Aroma threshold values | Aroma characteristics at 1.0%: phenolic, medicinal, sweet spicy, smoky with a methyl salicylate nuance. | | Taste threshold values | Taste characteristics at 2.0 ppm: sweet medicinal, phenolic and tarlike. | | Synthesis Reference(s) | Tetrahedron Letters, 30, p. 5215, 1989 DOI: 10.1016/S0040-4039(01)93745-1
Chemical and Pharmaceutical Bulletin, 27, p. 816, 1979 DOI: 10.1248/cpb.27.816
Journal of the American Chemical Society, 107, p. 2571, 1985 DOI: 10.1021/ja00294a073 | | 一般的な説明 | Colorless or yellow to brown-yellow or pinkish colored liquid with a phenol-like odor. Toxic by ingestion and/or skin absorption. May have a flash point between 100 and 199°F. Causes burns to skin, eyes and mucous membranes. Insoluble in water. | | 空気と水の反応 | Sensitive to light and air. Insoluble in water. | | 反応プロフィール | o-Cresol is incompatible with oxidizing agents and bases. Mixing o-Cresol with chlorosulfonic acid, nitric acid and oleum in a closed contained caused the temperature and pressure to increase. | | 危険性 | Questionable carcinogen. | | 健康ハザード | The chemical is rated as a very toxic compound with a probable oral lethal dose in humans of 50-500 mg/kg, or between 1 teaspoon and 1 ounce for a 70 kg (150 lb.) person. It is a strong dermal irritant and frequently causes dermatitis. Serious or fatal poisoning may result if large areas of skin are wet with cresol, o- and the substance is not removed immediately. Ingestion of even a small amount may cause paralysis and coma. It is corrosive to body tissues, with toxicity similar to phenol. | | 火災危険 | Fire may produce irritating or poisonous gases. Runoff from fire control water may give off poisonous gases. o-Cresol may burn but does not ignite readily. Container may explode in heat of fire. Slight explosion and fire hazard in the form of vapor when exposed to heat or flame. When heated to decomposition, o-Cresol emits highly toxic fumes. Reacts violently with nitric acid, oleum, and chlorosulfonic acid. Hazardous polymerization may not occur. | | 燃焼性と爆発性 | Not classified | | 安全性プロファイル | Poison by ingestion,
inhalation, subcutaneous, intravenous, and
intraperitoneal routes. Moderately toxic by
skin contact. A severe eye and skin irritant.
Human mutation data reported.
Questionable carcinogen with experimental
neoplastigenic data. Flammable when
exposed to heat, flame, or oxidants. To fight
fire, water may be used to blanket fire; foam,
fog, mist, dry chemical. See also other cresol
entries and PHENOL. | | 職業ばく露 | Cresol is used as a disinfectant and fumigant; as an ore flotation agent, and as an intermediate in the manufacture of chemicals, dyes, plastics, and antioxidants. A mixture of isomers is generally used; the concentrations of the components are determined by the source of the cresol. | | 概要 | 2-メチルフェノールとも呼ばれる。
常温にて無色の固体であり、他の化学物質の製造時によく使用されます。
フェノールの誘導体であり、p-クレゾールとm-クレゾールの異性体です。 | | Source | Detected in distilled water-soluble fractions of 87 octane gasoline (6.61 mg/L), 94 octane
gasoline (0.57 mg/L), Gasohol (1.17 mg/L), No. 2 fuel oil (2.64 mg/L), jet fuel A (0.72 mg/L),
diesel fuel (1.36 mg/L), and military jet fuel JP-4 (1.51 mg/L) (Potter, 1996). o-Cresol was also
detected in 82% of 65 gasoline (regular and premium) samples (62 from Switzerland, 3 from
Boston, MA). At 25 °C, concentrations were from 1.1–99 mg/L in gasoline and 70–6,600 μg/L in
water-soluble fractions. Average concentrations were 18 mg/L in gasoline and 1.2 mg/L in watersoluble
fractions (Schmidt et al., 2002).
A high-temperature coal tar contained 2-methylphenol at an average concentration of 0.25 wt %
(McNeil, 1983).
Occurs naturally in white sandlewood, sour cherries, peppermint leaves (1–10 ppb), tarragon,
asparagus shoots, tea leaves, coffee beans, Japanese privet, tomatoes, licorice roots, and African
palm oil (Duke, 1992).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rates of 2-methylphenol were 89.6 mg/kg of pine burned, 47.7 mg/kg of oak burned, and 37.8
mg/kg of eucalyptus burned. The particle-phase emission rates were 0.018 mg/kg of oak burned
and 0.006 mg/kg of eucalyptus burned. | | 環境運命予測 | Biological. Bacterial degradation of 2-methylphenol may introduce a hydroxyl group producing
3-methylcatechol (Chapman, 1972). In phenol-acclimated activated sludge, metabolites identified
include 3-methylcatechol, 4-methylresorcinol, methylhydroquinone, α-ketobutyric acid, dihydroxybenzaldehyde,
and trihydroxytoluene (Masunaga et al., 1986).
Chloroperoxidase, a fungal enzyme isolated from Caldariomyces fumago, reacted with 2-
methylphenol forming 2-methyl-4-chlorophenol (38% yield) and 2-methyl-6-chlorophenol
(Wannstedt et al., 1990).
Heukelekian and Rand (1955) reported a 5-d BOD value of 1.70 g/g which is 67.5% of the
ThOD value of 2.72 g/g. In activated sludge inoculum, 95.0% COD removal was achieved. The average rate of biodegradation was 54.0 mg COD/g?h (Pitter, 1976).
Soil. In laboratory microcosm experiments kept under aerobic conditions, half-lives of 5.1 and
1.6 d were reported for 2-methylphenol in an acidic clay soil (<1% organic matter) and slightly
basic sandy loam soil (3.25% organic matter) (Loehr and Matthews, 1992).
Surface Water. In river water, the half-life of 2-methylphenol was 2 and 4 d at 20 and 4 °C,
respectively (Ludzack and Ettinger, 1960).
Groundwater. Nielsen et al. (1996) studied the degradation of 2-methylphenol in a shallow,
glaciofluvial, unconfined sandy aquifer in Jutland, Denmark. As part of the in situ microcosm
study, a cylinder that was open at the bottom and screened at the top was installed through a cased
borehole approximately 5 m below grade. Five liters of water was aerated with atmospheric air to
ensure aerobic conditions were maintained. Groundwater was analyzed weekly for approximately
3 months to determine 2-methylphenol concentrations with time. The experimentally determined
first-order biodegradation rate constant and corresponding half-life were 0.2/d and 3.5 d,
respectively. Groundwater contaminated with phenol and other phenols degraded in a
methanogenic aquifer to methane and carbon dioxide. These results could not be duplicated in the
laboratory utilizing an anaerobic digester (Godsy et al., 1983).
Photolytic. Sunlight irradiation of 2-methylphenol and nitrogen oxides in air yielded the
following gas-phase products: acetaldehyde, formaldehyde, pyruvic acid, peroxyacetyl nitrate,
nitrocresols, and trace levels of nitric acid and methyl nitrate. Particulate phase products were also
identified and these include 2-hydroxy-3-nitrotoluene, 2-hydroxy-5-nitrotoluene, 2-hydroxy-3,5-
dinitrotoluene, and tentatively identified nitrocresol isomers (Grosjean, 1984). Absorbs UV light
at a maximum wavelength of 270 nm (Dohnal and Fenclová, 1995).
Chemical/Physical. Ozonation of an aqueous solution containing 2-methylphenol (200 to 600
mg/L) yielded formic, acetic, propionic, glyoxylic, oxalic, and salicylic acids (Wang, 1990). In a
different experiment, however, an aqueous solution containing 2-methylphenol (1 mM) reacted
with ozone (11.7 mg/min) forming 2-methylmuconic acid and hydrogen peroxide as end products.
The proposed pathway of degradation involved electrophilic aromatic substitution by the first
ozone molecule followed by a 1,3-dipolar addition of the second ozone molecule to the cleaved
ring (Beltran et al., 1990). | | 輸送方法 | UN2076 Cresols, liquid, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material. UN3455 Cresols, solid, Hazard class: 6.1; Labels: 6.1- Poisonous materials, 8-Corrosive material. | | 純化方法 | It can be freed from m-and p-isomers by repeated fractional distillation, It crystallises from *benzene by addition of pet ether. It has been fractionallly crystallised by partial freezing of its melt. The 3,5-dinitrobenzoate (prepared with 3,5-dinitrobenzoyl chloride in dry pyridine, and recrystallised from EtOH or aqueous Me2CO) has m 138o. [Beilstein 6 IV 1940.] | | 不和合性 | Vapors may form explosive mixture with air. Incompatible with strong acids; oxidizers, alkalies, aliphatic amines; amides, chlorosulfonic acid; oleum. Decomposes on heating, producing strong acids and bases, causing fire and explosion hazard. Liquid attacks some plastics and rubber. Attacks many metals. | | 廃棄物の処理 | Wastewaters may be subjected to biological treatment. Concentrations may be further reduced by ozone treatment. High concentration wastes may be destroyed in special waste incinerators. |
|