| | BUPRENORPHINE HYDROCHLORIDE Basic information |
| Product Name: | BUPRENORPHINE HYDROCHLORIDE | | Synonyms: | Zalban;[5α,7α(S)]-17-(Cyclopropylmethyl)-α-(1,1-dimethylethyl)-4,5-epoxy-18,19-dihydro-3-hydroxy-6-methoxy-α-methyl-6,14-ethenomorphinan-7-methanolhydrochloride;BUPRENORPHINE HCL;BUPRENORPHINE HYDROCHLORIDE;Buprenorphine Hydrochloride (CIII), USP;Buprenorphine Hydrochloride CIII (50 mg);6,14-EthenoMorphinan-7-Methanol,17-(cyclopropylMethyl)-a-(1,1-diMethylethyl)-4,5-epoxy-18,19-dihydro-3-hydroxy-6-Methoxy-a-Methyl-, hydrochloride (1:1);6,14-Ethenomorphinan-7-methanol, 17-(cyclopropylmethyl)-α-(1,1-dimethylethyl)-4,5-epoxy-18,19-dihydro-3-hydroxy-6-methoxy-α-methyl-, hydrochloride, [5α,7α(S)]- | | CAS: | 53152-21-9 | | MF: | C28H40ClNO4 | | MW: | 490.07 | | EINECS: | 258-396-8 | | Product Categories: | Amines;Chiral Reagents;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 53152-21-9.mol | 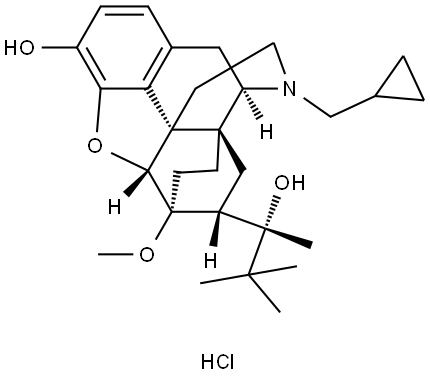 |
| | BUPRENORPHINE HYDROCHLORIDE Chemical Properties |
| Melting point | 260-262?C (dec.) | | storage temp. | -0°C | | solubility | Sparingly soluble in water, freely soluble in methanol, soluble in ethanol (96 per cent), practically insoluble in cyclohexane. | | form | Powder | | Water Solubility | Soluble to 25 mM in water and to 50 mM in ethanol |
| | BUPRENORPHINE HYDROCHLORIDE Usage And Synthesis |
| Description | Buprenorphine (hydrochloride) (CRM) (Item No. ISO60178) is a certified reference material that is structurally categorized as an opioid. It is a partial agonist of the μ-opioid receptor (Ki = 4.18 nM) that less potently acts at δ- and κ-opioid receptors (Kis = 25.8 and 12.9 nM, respectively). Buprenorphine is used, alone or with naloxone (Item Nos. ISO60194 | 15594), to counter opiate addiction. This product is intended for research and forensic applications. | | Chemical Properties | White Solid | | Uses | Controllled substance (narcotic). Analgesic that demonstrates narcotic agonist-antagonist properties. | | Definition | ChEBI: The hydrochloride salt of buprenorphine. | | Brand name | Buprenex (Reckitt Benckiser); Subutex
(Reckitt Benckiser). | | Acquired resistance | Buprenorphine is 20 to 50 times more potent than morphine in producing an ED50 analgesic effect
in animal studies; however, it cannot produce an ED100 (compared to morphine) in these tests.
Thus, buprenorphine is a potent partial agonist at μ opioid receptors. It also is a partial agonist at κreceptors but more of an antagonist at δ receptors. Buprenorphine, at 0.4 mg intramuscular dose,
will produce the same degree of analgesia as 10 mg of morphine. Because of its partial agonist
properties, it has a lower ceiling on its analgesic action but also produces less severe respiratory
depression. It is incapable of producing tolerance and addiction comparable to full μ agonists. In
fact, buprenorphine's partial agonist action, very high affinity for opioid receptors, and high
lipophilicity combine to give buprenorphine a tolerance, addiction, and withdrawal profile that is
unique among the opioids. When given by itself to opioid-naive patients, little tolerance or addictive
potential (Schedule 5) is observed. A mild withdrawal can occur some 2 weeks after the last dose
of buprenorphine. Buprenorphine will precipitate withdrawal symptoms in highly addicted
individuals, but it will suppress symptoms in individuals who are undergoing withdrawal from
opioids. It effectively blocks the effect of high doses of heroin. Because of these properties,
buprenorphine has been approved for office-based use in treating opioid dependence. It also
has been reported to suppress cocaine use and addiction. | | Biological Activity | ORL 1 receptor agonist that also displays mixed antagonist/partial agonist activity at κ , δ and μ -opioid receptors. | | Biochem/physiol Actions | Full agonist at ORL1 receptors, antagonist or partial agonist at μ, κ, and δ opioid receptors. | | Clinical Use | Buprenorphine undergoes extensive first-pass 3-O-glucuronidation, which negates its usefulness
after oral dose. It is available in parenteral and sublingual dosage forms. The typical dose is 0.3 to
0.6 mg three times per day by intramuscular injection for analgesia or 8 mg/day as a sublingual
tablet for opioid-dependence maintenance. The duration of analgesic effect is 4 to 6 hours. After
parenteral dose, approximately 70% of the drug is excreted in the feces, and the remainder
appears as N-dealkylated and conjugated metabolites in the urine.
Naloxone is not an effective antagonist to buprenorphine because of the latter's high binding
affinity to opioid receptors. | | storage | Store at RT |
| | BUPRENORPHINE HYDROCHLORIDE Preparation Products And Raw materials |
|