| 化学名: | 四酸化二窒素 | | 英語化学名: | Nitrogen Tetroxide | | 别名: | Distickstofftetroxid;N2O4;Nitrogen dioxide, di-;Nitrogen oxide (N2O4);nitrogenoxide(n2o4);nitrogentetraoxide;nitrogentetroxide,liquid;NITROGEN PEROXIDE | | CAS番号: | 10544-72-6 | | 分子式: | N2O4 | | 分子量: | 92.01 | | EINECS: | 234-126-4 | | カテゴリ情報: | | | Mol File: | 10544-72-6.mol | 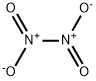 |
| 融点 | −11 °C(lit.) | | 沸点 | 21 °C(lit.) | | 比重(密度) | 2.62 g/mL at 25 °C(lit.) | | 蒸気密度 | 1.58 (21 °C, vs air) | | 蒸気圧 | 14.33 psi ( 20 °C) | | 溶解性 | reacts with H2O | | 外見 | gas | | 色 | colorless liquid; equil with
NO | | 水溶解度 | reac H2O [CRC10] | | Dielectric constant | 2.5(15℃) | | EPAの化学物質情報 | Nitrogen tetroxide (10544-72-6) |
| 主な危険性 | T+ | | Rフレーズ | 26-34 | | Sフレーズ | 9-26-28-36/37/39-45 | | RIDADR | UN 1067 2.3 | | WGK Germany | 1 | | RTECS 番号 | QX1575000 | | HSコード | 28112900 | | 有毒物質データの | 10544-72-6(Hazardous Substances Data) | | 毒性 | dog,LC50,inhalation,260mg/m3 (260mg/m3),CARDIAC: OTHER CHANGESLUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMABEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD,"Spravochnik po Toksikologii i Gigienicheskim Normativam Vol. -, Pg. 6, 1999. |
| | 四酸化二窒素 Usage And Synthesis |
| 解説 | N2O4(92.01).四酸化二窒素は,二酸化窒素を冷却すると無色の固体として得られる.融点-9.3 ℃.液体は淡黄色であるが,この色は二酸化窒素の存在による.沸点21.2 ℃ では約1% のNO2を含む.気体は高温になるほどNO2が増し,100 ℃ で約90% NO2となる.アルカリ金属と反応して硝酸塩と一酸化窒素を生じる.液体はNO+とNO3-に分解しやすい.森北出版「化学辞典(第2版) | | 用途 | 酸化反応時の触媒、アクリル誘導体蒸留時の重合防止剤、ロケット推進薬原料 | | 用途 | 四酸化二窒素は,気体状態では二酸化窒素と平衡混合物になり、淡黄色を呈する。ロケット燃料の酸化剤などに用いられる。液体N2O4は無水硝酸塩をつくるときの溶媒,宇宙機ヒドラジン燃料の酸化剤などに用いられる.大気汚染物質(NOx)の一種である. | | 説明 | Nitrogen tetraoxide is primarily known for its use as a rocket
propellant and became an oxidizer of choice by the late 1950s.
Nitrogen tetraoxide has been used as a propellant for missiles
as well as for spacecraft beginning with its use in Titan rockets
in the late 1950s. | | 化学的特性 | col liquid [CRC10] | | 物理的性質 | Colorless liquid or gas; exists in equilibrium with NO2; density 1.45 g/mL at 20°C; boils at 21.25°C; freezes at -9.35°C to a colorless diamagnetic solid; critical temperature 157.85°C; critical pressure 99.64 atm; critical volume 167 cm3/mol; reacts with water. | | 使用 | Nitrogen tetroxide is a solvent and a powerful and selective oxidizing agent. It’s adducts with organic solvents are used to synthesize nitrates of noble metals. | | 使用 | Nitrogen tetraoxide is formed by pressurizing liquid nitrogen
dioxide. It is a gas at normal temperature and pressure, and is
used in the manufacture of explosives and rocket fuels.
Nitrogen tetraoxide is used as a catalyst in oxidation reactions
and in many other industrial applications. It is also a component
of nitric and sulfuric acid. | | 定義 | dinitrogen tetroxide: A colourlessto pale yellow liquid or a brown gas,N2O4; r.d. 1.45 (liquid); m.p. –11.2°C;b.p. 21.2°C. It dissolves in water withreaction to give a mixture of nitricacid and nitrous acid. It may be readilyprepared in the laboratory by thereaction of copper with concentratednitric acid; mixed nitrogen oxidescontaining dinitrogen oxide may alsobe produced by heating metal nitrates.The solid compound is whollyN2O4 and the liquid is about 99%N2O4 at the boiling point; N2O4 is diamagnetic.In the gas phase it dissociatesto give nitrogen dioxideN2O4?2NO2Because of the unpaired electron thisis paramagnetic and brown. LiquidN2O4 has been widely studied as anonaqueous solvent system (selfionizesto NO+ and NO3-). Dinitrogentetroxide, along with other nitrogenoxides, is a product of combustionengines and is thought to be involvedin the depletion of stratosphericozone. | | 定義 | A colorless gas that becomes a pale yellow
liquid below 21°C and solidifies below
–11°C. On heating, the gas dissociates to
nitrogen dioxide molecules:
N2O4(g) = 2NO2(g)
This dissociation is complete at 140°C.
Liquid dinitrogen tetroxide has good solvent
properties and is used as a nitrating
agent. | | 製造方法 | Nitrogen tetroxide always is formed along with nitrogen dioxide during preparation of the dioxide (See Nitrogen Dioxide.) Mixed oxides are produced by oxidation of nitric oxide (NO) in air, heating metal nitrates, or by metals reacting with nitric acids or nitrates. | | 一般的な説明 | Red-brown liquid with a sharp, unpleasant chemical odor. Low-boiling (boiling point 21.15°C) and held as a liquid by compression. Density 1.448 g / cm3. Consists of an equilibrium mixture of brown NO2 (nitrogen dioxide) and colorless N2O4 (diNITROGEN DIOXIDE). Evolves poisonous brown vapors. Cylinders and ton containers may not be equipped with a safety relief device. Prolonged exposure of the containers to fire or heat may result in their violent rupturing and rocketing. | | 空気と水の反応 | Reacts with water to form nitric acid and nitric oxide. | | 反応プロフィール | Liquid NITROGEN DIOXIDE is an oxidizing agent consisting of an equilibrium mixture of colorless dinitrogen tetraoxide (N2O4) and red-brown nitrogen dioxide (NO2). The exact composition of the mixture depends on the temperature with higher temperature favoring conversion to NO2. Vaporizes readily to give NO2, also an oxidizing agent. Noncombustible but can accelerate the burning of combustible materials. Reacts with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). Reacts with alkalis to form nitrates and nitrites [Merck 11th ed. 1989]. Corrodes steel if wet, but can be stored in steel cylinders if dry [Merck]. Reacts explosively with liquid ammonia even at very low temperatures (below its freezing point) [Mellor, 1940, Vol. 8, 54]. Reacts energetically with boron trichloride [Mellor, 1946, Vol. 5, 132]. Mixtures with metal carbonyls are hypergolic (enflame immediately). Mixtures with halocarbons, hydrazine derivatives, heterocyclic bases (pyridine), isopropyl nitrite/propyl nitrite, active metals (magnesium, calcium, etc.), nitroaromatics, nitrogen trichloride, phosphorus, triethylamine, unsaturated hydrocarbons may react explosively. Accidental mixing with hot cyclohexane caused an explosion [MCA Case History 128. 1962]. A mixture with acetonitrile and indium showed no evidence of change for a time and then detonated when shaken (ascribed to the catalyzed oxidation of acetonitrile) [Chem. & Ind., 1958, 1004]. Mixture with alcohols produced a violent explosion [Chem. Eng. News, 1955, 33, 2372]. Vapor reacts with barium oxide incandescently [Mellor, 1940, Vol. 8, 545]. A slow reaction between the vapor and formaldehyde became explosive near 180°C [Trans. Faraday Soc. 45:767-770. 1949]. Manganese and potassium both ignite in the vapor [Ann. Chem. et Phys.(2) 2:317]. The vapor and ozone react with the evolution of light and often explode when mixed [J. Chem. Phys. 18:366. 1920]. | | 危険性 | See Nitrogen Dioxide, Hazard. | | 健康ハザード | Very concentrated fumes produce coughing, choking, headache, nausea, pain in chest and abdomen; otherwise, few symtoms appear at time of exposure. After symptom-free period of 5-72 hours, pulmonary edema gradually develops, causing fatigue, restlessness, coughing, difficulty in breathing, frothy expectoration, mental confusion, lethargy, bluish skin, and weak, rapid pulse. Since NOX interferes with gas exchange in lungs, unconscious- ness and death by asphyxiation can result, usually within a few hours after onset of pulmonary edema. | | 燃焼性と爆発性 | Non flammable | | 安全性プロファイル | A poison. Moderately
toxic by inhalation. When heated to
decomposition it emits toxic fumes of NOx.
See also NITROGEN MONOXIDE. | | 環境運命予測 | Nitrogen tetroxide is released into the atmosphere where it can
undergo reactions, leading to air pollution. | | 純化方法 | Purify it by oxidation at 0o in a stream of oxygen until the blue colour changes to red-brown. Alternatively distil it from P2O5, then solidify it by cooling in a deep-freeze (at –78o, giving nearly colourless crystals). Oxygen can be removed by alternate freezing and melting. TOXIC VAPOUR. [Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 488-489 1963.] | | Toxicity evaluation | Nitrogen tetraoxide is absorbed through the respiratory system
and reacts with blood, reducing fluid levels, inducing massive
pulmonary edema and a severe reduction in hemoglobin levels. |
|