|
ChemicalBook Optimization Suppliers |
| 名前: |
Giant chemicals Gold |
| 電話番号: |
028-85434334 18108076537 |
| 電子メール: |
market@gianthx.com |
|
| 化学名: | 2,3-ジクロロ安息香酸 | | 英語化学名: | 2,3-Dichlorobenzoic acid | | 别名: | Lamotrigine Related Compound B (USP);2,3Dichlorobenzoic acid (Lamotrigine Related Compound B)
;RARECHEM AL BO 0323;TIMTEC-BB SBB003641;2,3-DICHLOROBENZOIC ACID;2,3-DICHLOROBENZOIC ACID PESTANAL, 250 M;2,3-Dichlorobenzoicacid,98%;Benzoic acid, 2,3-dichloro- | | CAS番号: | 50-45-3 | | 分子式: | C7H4Cl2O2 | | 分子量: | 191.01 | | EINECS: | 200-039-5 | | カテゴリ情報: | Alpha sort;D;DAlphabetic;DIA - DIC;Pesticides&Metabolites;C7;Carbonyl Compounds;Carboxylic Acids;intermediate;acid;Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts;Benzoic acid;Organic acids | | Mol File: | 50-45-3.mol | 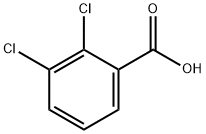 |
| 融点 | 168-170 °C (lit.) | | 沸点 | 273.68°C (rough estimate) | | 比重(密度) | 1.4410 (rough estimate) | | 屈折率 | 1.4590 (estimate) | | 貯蔵温度 | Sealed in dry,Room Temperature | | 溶解性 | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | | 酸解離定数(Pka) | 2.53±0.25(Predicted) | | 色 | White to Off-White | | 水溶解度 | slightly soluble | | BRN | 1946217 | | InChI | InChI=1S/C7H4Cl2O2/c8-5-3-1-2-4(6(5)9)7(10)11/h1-3H,(H,10,11) | | InChIKey | QAOJBHRZQQDFHA-UHFFFAOYSA-N | | SMILES | C(O)(=O)C1=CC=CC(Cl)=C1Cl | | CAS データベース | 50-45-3(CAS DataBase Reference) | | EPAの化学物質情報 | 2,3-Dichlorobenzoic acid (50-45-3) |
| | 2,3-ジクロロ安息香酸 Usage And Synthesis |
| 外観 | 白色~ほとんど白色粉末~結晶 | | 化学的特性 | white to light yellow crystal powder | | 使用 | 2,3-Dichlorobenzoic acid is the key intermediate for antiepileptic drug Lamotrigine (L173250) and other pharmaceuticals for the treatment of central nervous system (CNS) disorders and disease. | | 定義 | ChEBI: 2,3-dichlorobenzoic acid is a chlorobenzoic acid that is benzoic acid in which the ring hydrogens at positions 2 and 3 are substituted by chloro groups. It has a role as an impurity and a bacterial xenobiotic metabolite. It is a chlorobenzoic acid and a dichlorobenzene. It is functionally related to a benzoic acid. It is a conjugate acid of a 2,3-dichlorobenzoate. | | 一般的な説明 | 2,3-Dichlorobenzoic acid is an intermediate metabolite of polychlorinated biphenyls (PCBs). | | 合成 | 2,3-Dichlorobenzoic acid was prepared from 2,3-dichloroaniline by diazotization and Myerwine reaction to 2,3-dichlorobenzaldehyde, and then oxidized by potassium permanganate, with an overall yield of 45%.
Another synthesis of 2,3-dichlorobenzoic acid:
Step (a) A solution of 2,3-dichloroiodobenzene in sodium dried ether was added dropwise to magnesium turnings and a crystal of iodine with warming so as to form a Grignard reagent.
Step (b) The mixture of Step (a) was stirred and refluxed then cooled and transferred dropwise, under nitrogen, into a stirred mixture of sodium dried ether containing solid carbon dioxide.
Step (c) The mixture of Step (b) was stirred for 2 hours, left overnight to warm to room temperature, then treated with ice and aqueous hydrochloric acid, and the product extracted with ether. The combined ether extracts were washed with water then repeatedly extracted with an aqueous sodium hydroxide. These basic solutions were combined, stirred with activated charcoal for 10 minutes, filtered and the cooled filtrate was acidified with concentrated hydrochloric acid at lO.degree. C.
Step (d) The resultant solid was filtered off, washed with water (2.times.20 mis) and dried in vacuo. Yield 77.6%. | | 純化方法 | Aromatic acid impurities (to <0.05%) can be removed via the (±)-�-methylbenzylamine salt as described for 2,4-dichlorobenzoic acid [Ley & Yates Organic Process Research & Development 12 120 2008.] Crystallise the acid from H2O, aqueous EtOH, or 30% aqueous AcOH and several times from *C6H6, then dry it in vacuo at 40o overnight. The methyl ester has m 35-39o . [Hope & Riley J Chem Soc 123 2478 1923, Lock Monatsh Chem 90 687 1959, Shorter & Mather J Chem Soc 4744 1961, Beilstein 9 II 228, 9 IV 998.] |
|