|
ChemicalBook Optimization Suppliers |
|
| 融点 | 218-220° | | 沸点 | 742.8±60.0 °C(Predicted) | | 比重(密度) | 1.448±0.06 g/cm3(Predicted) | | 貯蔵温度 | Keep in dark place,Inert atmosphere,Room temperature | | 溶解性 | Soluble in DMSO | | 酸解離定数(Pka) | 9.31±0.10(Predicted) | | 色 | White to Light yellow to Light orange | | Merck | 14,7996 | | InChI | InChI=1S/C16H20N2O4S2/c1-9-15(21)13(5-19)11(3-17-9)7-23-24-8-12-4-18-10(2)16(22)14(12)6-20/h3-4,19-22H,5-8H2,1-2H3 | | InChIKey | SIXLXDIJGIWWFU-UHFFFAOYSA-N | | SMILES | S(CC1=C(CO)C(O)=C(C)N=C1)SCC1=C(CO)C(O)=C(C)N=C1 | | CAS データベース | 1098-97-1(CAS DataBase Reference) |
| 主な危険性 | Xi | | Rフレーズ | 36/37/38 | | Sフレーズ | 26 | | WGK Germany | 2 | | RTECS 番号 | UT4836666 | | HSコード | 2936.25.0000 |
| | ピリチオキシン Usage And Synthesis |
| 外観 | 白色~うすい黄色~うすい黄赤色粉末~結晶 | | 効能 | 向知性薬 | | 説明 | Pyritinol, the disulfide of pyridoxine-5-thiol,
is marketed as a cerebral stimulant in Europe.
Both the thiol and the disulfide have been shown
to have d-penicillamine-like activity in the treatment of rheumatoid arthritis. Adverse effects are
similar to those seen with d-penicillamine. Occasionally, pyritinol seems effective and better
tolerated in some individuals who fail to respond
to d-penicillamine. | | 使用 | cognition enhancer, nootropic | | 使用 | Pyritinol can be used as a nootropic used in the prevention and treatment of cerebrovascular diseases. | | 定義 | ChEBI: Pyritinol is a member of methylpyridines. | | brand name | Biontabol;Bonifwn;Cefalogen;Cerebrotrofina;Cervitalin;Divalvon-d;Enbol;Encefort;Encerbrovit;Encerebron;Fulneurina;Geribolina;Gerontabol comp.;Juniormen;Leonar;Logos;Maind;Musa;Neuroxin;Piriditol;Piririomin;Piritinol;Piritiomin;Plenumil;Sawaxin;Scintidin;Tibased;Tomevit;Tonobrein;Tonomentis. | | 世界保健機関(WHO) | Pyritinol, which is claimed to promote the uptake of glucose in
the brain, is used in the treatment of cerebrovascular disorders. However, WHO is
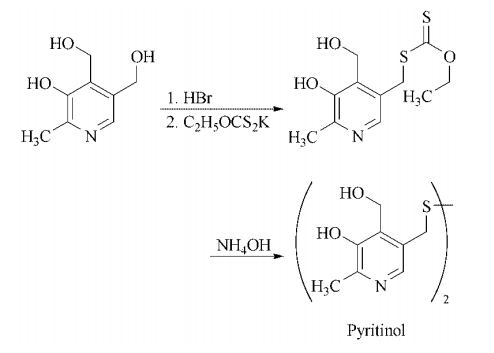
not aware of controlled experimental data to show that it has any therapeutic effect. | | 合成 | Synthesis: treatment of pyridoxine with hydrobromic acid gives 4,5-bis(bromomethyl)-3-
hydroxy-2-methylpyridinium bromide, which
when treated in the cold with potassium
ethyl xanthate gives ethyl 4-hydroxymethyl-
3-hydroxy-2-methyl-3-pyridyl methylxanthate.
Hydrolysis and oxidation are carried out in
aqueous-alcoholic ammonia to give the disulfide
pyritinol.
 |
|