|
ChemicalBook Optimization Suppliers |
|
| 融点 | 152-156 °C | | 比旋光度 | 101 º (c=1, dioxane 25 ºC) | | 沸点 | 370.65°C (rough estimate) | | 比重(密度) | 1.0484 (rough estimate) | | 屈折率 | 1.4709 (estimate) | | 闪点 | 5 °C | | 貯蔵温度 | 2-8°C | | 溶解性 | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: soluble18.2 mg/ml | | 酸解離定数(Pka) | 15.06±0.60(Predicted) | | 外見 | powder | | 水溶解度 | 22.79mg/L(20 ºC) | | Merck | 13,9255 | | BRN | 1915399 | | 安定性: | Stable. Combustible. Incompatible with strong oxidizing agents. | | InChIKey | MUMGGOZAMZWBJJ-DYKIIFRCSA-N | | CAS データベース | 58-22-0(CAS DataBase Reference) | | NISTの化学物質情報 | Testosterone(58-22-0) | | EPAの化学物質情報 | Testosterone (58-22-0) |
| | テストステロン Usage And Synthesis |
| 外観 | 白色~わずかにうすい黄色、結晶~粉末 | | 解説 | (17β)-hydroxyandrost-4-en-3-one.C19H28O2(288.42).男性ホルモンの一つ.1935年,E. Laqueurらにより,ウシの睾丸から単離された.テストステロンは,睾丸の間質細胞からおもに分泌されるが,副腎皮質の網状層の細胞でも合成される前立腺や精嚢に多く分布している.1935年,A.F. Butenandt(ブテナント),L. Ruzicka(ルジチカ)らにより,デヒドロエピアンドロステロンより合成された.白色~微黄色の結晶または結晶性粉末.融点153~157 ℃.[α]20D+101~+105°(ジオキサン).UVλmax 238 nm.水に難溶,無水エタノールまたはクロロホルムに可溶.ジオキサンにやや溶けやすく,エーテルまたはごま油に溶けにくい.ヒトの血中濃度は男0.64 μg/dL,女0.034 μg/dL である.テストステロンは真の男性ホルモンとされており,前立腺や精嚢などの副性器および陰茎,陰嚢などの外性器の発育,さらに第二次性徴の発育にも重要な役割を果たしている.また,タンパク同化作用が強い.男性性器機能不全症,乳がん,更年期障害の治療に用いられている.森北出版「化学辞典(第2版) | | 用途 | アンドロゲン受容体作動薬です。 薬物代謝酵素CYP3A4の基質であり、薬物代謝試験で使用されます。 テストステロンは代謝されると6β-ヒドロキシテストステロンに変換されます。
| | 効能 | 男性ホルモン補充薬, アンドロゲン受容体作動薬 | | 説明 | Testosterone (Item No.15645) is an analytical reference material categorized as an anabolic androgenic steroid. Testosterone is an endogenous metabolite of androstenedione (Item Nos. ISO60161 | 15874) and estradiol (Item Nos. ISO60155 | 10006315). Anabolic steroids, including testosterone, have been used to enhance physical performance in athletes. Testosterone is regulated as a Schedule III compound in the United States. This product is intended for research and forensic applications. | | 説明 | Testosterone (CRM) (Item No. ISO60154) is a certified reference material categorized as an anabolic androgenic steroid. Testosterone is an endogenous metabolite of androstenedione (Item Nos. ISO60161 | 15874) and estradiol (Item Nos. ISO60155 | 10006315). Anabolic steroids, including testosterone, have been used to enhance physical performance in athletes. Testosterone is regulated as a Schedule III compound in the United States. This product is intended for research and forensic applications. | | 化学的特性 | white crystalline odourless solid | | 使用 | Secreted by the testis and is converted to dihydrotestosterone in the target tissue where is appears to mediate many of the biological actions of testosterone.
CONTROLLED SUBSTANC | | 使用 | androgen, antineoplastic | | 使用 | Testosterone secreted by the testis is converted to dihydrotestosterone in the target tissues where it appears to mediate many of the biological actions of testosterone. Androgens direct the development of the male phenotype during embryogenesis and at puberty. | | 使用 | Testosterone, Principal hormone of the testes, produced by the interstitial cells. Major circulating androgen; converted by 5α-reductase in androgen-dependent target tissues to 5α-dehydrotestosterone
which is required for normal male sexual differentiation. Also converted by aromatization to Estradiol.
Testerone is a controlled substance (anabolic steroid). Androgen. | | 使用 | Rivastigmine metabolite | | 定義 | ChEBI: An androstanoid having 17beta-hydroxy and 3-oxo groups, together with unsaturation at C-41C-5.. | | 一般的な説明 | Testosterone, 17β-hydroxyandrost-4-en-3-one, is a naturally occurring androgen in men. Inwomen, it mainly serves as a biosynthetic precursor to estradiolbut also has other hormonal effects. It is rapidly metabolizedto relatively inactive 17-ones, however,preventing significant oral activity. Testosterone is availablein a transdermal delivery system (patch), a gel formulation, abuccal system, and as implantable pellets. | | 危険性 | A confirmed carcinogen. | | 健康ハザード | Controls secondary male sex characteristics Maintains functional competence of male reproductive ducts and glands
Increases protein anabolism; maintains spermatogenesis; inhibits follotropin
Increases male sex behavior; increases closure of epiphyseal plates | | 生物活性 | Endogenous androgen receptor agonist. | | Biochem/physiol Actions | Testosterone secreted by the testis is converted to dihydrotestosterone in the target tissues where it appears to mediate many of the biological actions of testosterone. Androgens direct the development of the male phenotype during embryogenesis and at puberty. | | 安全性プロファイル | Confirmed carcinogen with experimental neoplastigenic and teratogenic data. Poison by intraperitoneal route. Human teratogenic effects by unspecified route: developmental abnormalities of the urogenital system. Experimental reproductive effects. Human mutation data reported. Workers engaged in manufacture and packagmg have shown effects from this hormone, e.g., enlargement of the breasts in male workers. A promoter. When heated to decomposition it emits acrid smoke and irritating fumes. Used as a drug for the treatment of hypogonadism and metastatic breast cancer. | | 合成 | Testosterone, 17|?-hydroxyandrost-4-ene-3-one (29.1.5), is made in a num�ber of ways from different substances, including cholesterol, although it is most often
made from androstenolone acetate. In order to do this, the keto-group at C17 of the steroid
system of androstenolone acetate is reduced to a hydroxyl group by either sodium boro�hydride, lithium aluminum hydride, or hydrogen over Raney nickel, all of which result in
a 17|?-hydroxy compound. In the given example, reduction by sodium borohydride or
hydrogen over Raney nickel leads to the formation of 3|?-acetoxy-5-androsten-17|?-ol
(29.1.1). The hydroxyl group resulting from reduction then undergoes acylation by ben�zoyl chloride in pyridine, which forms a diester (29.1.2). After that, taking into consider�ation the differences in the acidic region of the two ester groups present in the molecule as
well as the long-known fact that 17-hydroxy-group ester derivatives are harder to
hydrolyze than 3-hydroxy-group ester derivatives, the acetyl protection of the hydroxyl
group at C3 is removed by selective hydrolysis using potassium hydroxide in ethanol, and
the resulting alcohol (29.1.3) is oxidized to a ketone (29.1.4) by aluminum isopropylate in
the presence of cyclohexanone as a hydrogen acceptor, during which isomerization of the
double bond from position C5¨CC6 to C4¨CC5 simultaneously takes place. Subsequent hydrol�ysis of the remaining ester region of the molecule using an alkali gives the desired testos�terone (29.1.5) . When necessary to convert this into the corresponding ester
(propionate, enantate, cypionate, and a few other testosterone esters), the necessary acyla�tion can be accomplished. 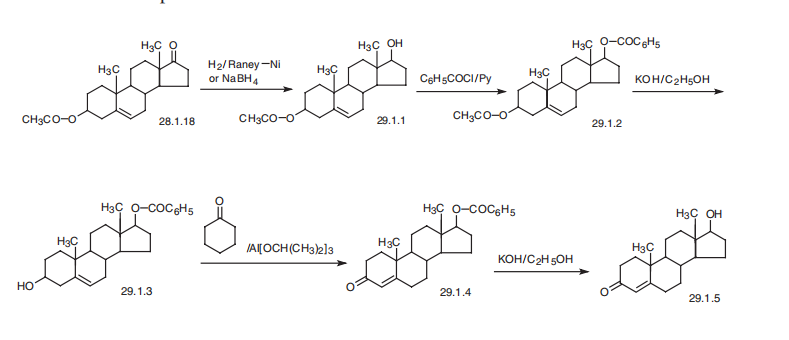
| | 純化方法 | Crystallise testosterone from aqueous acetone, hexane or isoPrOH. The long needles that separated from EtOH/AcOH were used for X-ray crystallography [Roberts et al. J Chem Soc Perkin Trans II 1978 1973.] The acetate [1045-69-8] crystallises from MeOH or aqueous Me2CO, with m 140-141o and [] D 20 +87.8o (c 1, EtOH). [Ruzicka et al. Helv Chim Acta 18 1478 1935 and 19 99, 842 1936, Beilstein 8 IV 974.] |
|