|
ChemicalBook Optimization Suppliers |
| 名前: |
|
| 電話番号: |
821-50328103-801 18930552037 |
| 電子メール: |
3bsc@sina.com |
| 名前: |
ChemShuttle, Inc. |
| 電話番号: |
0150-83588313-811 18800520310 |
| 電子メール: |
sales@chemshuttle.com |
|
| 化学名: | L-165041 | | 英語化学名: | L-165,041 | | 别名: | L-165041, >=98%;2-[4-[3-(4-acetyl-3-hydroxy-2-propylphenoxy)propoxy]phenoxy]-;2-(4-(3-(4-acetyl-3-hydroxy-2-propylphenoxy)propoxy)phenoxy)acetic acid;Acetic acid, 2-[4-[3-(4-acetyl-3-hydroxy-2-propylphenoxy)propoxy]phenoxy]-;L 1q5041;COMPOUND P;L-165,041;4-[3-(4-ACETYL-3-HYDROXY-2-PROPYLPHENOXY)PROPOXY]PHENOXYACETIC ACID | | CAS番号: | 79558-09-1 | | 分子式: | C22H26O7 | | 分子量: | 402.44 | | EINECS: | | | カテゴリ情報: | Intracellular receptor;API | | Mol File: | 79558-09-1.mol | 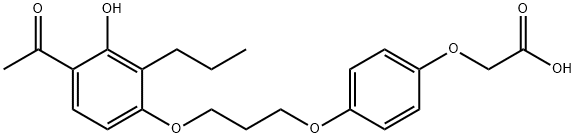 |
| 融点 | 127-128 °C(lit.) | | 沸点 | 600.8±55.0 °C(Predicted) | | 比重(密度) | 1.222±0.06 g/cm3(Predicted) | | 貯蔵温度 | 2-8°C | | 溶解性 | DMSO: >10 mg/mL | | 外見 | solid | | 酸解離定数(Pka) | 3.23±0.10(Predicted) | | 色 | off-white | | 安定性: | Light Sensitive |
| | L-165041 Usage And Synthesis |
| 使用 | L-165,041 has been used as a peroxisome proliferator-activated receptor β/δ (PPARβ/δ) ligand to study its influence on PPARβ/δ mediated postnatal myogenesis in C2C12 myoblasts. | | 定義 | ChEBI: 2-[4-[3-(4-acetyl-3-hydroxy-2-propylphenoxy)propoxy]phenoxy]acetic acid is an aromatic ketone. | | 一般的な説明 | A cell-permeable phenoxyacetic acid derivative that acts as a potent and selective peroxisome proliferator activator receptor δ (PPARδ) agonist (Ki = 6 nM for hPPARδ and 730 nM for hPPARγ). Potently induces adipocyte differentiation in NIH-PPARδ cells at 500 nM and raises total cholesterol in insulin resistant db/db mice without altering glucose or triglycerides levels. Increases UCP3 (uncoupling protein 3) gene expression in L6 myotubes. Inhibits cytokine-induced expression of VCAM-1 (vascular cell adhesion molecule-1) and the secretion of MCP-1 (monocyte chemotactic protein-1) in EAhy926 cells. | | 生物活性 | Potent PPAR δ agonist (K i = 6 nM); displays > 100-fold selectivity for both mouse and human PPAR δ ? receptors over other subtypes. In vivo, raises plasma cholesterol levels in insulin-resistant db/db mice and is neuroprotective in models of cerebral infarction and Parkinson's disease. | | Biochem/physiol Actions | Cell permeable: yes | | in vitro | l-165041, which is a selective and potent pparδligand, displayed in this specified transactivation system, apart from its highly efficacious pparδ agonist activity, partial and full agonism at, respectively, pparγ2 and pparαsubtypes [1]. | | in vivo | l-165041 could drastically reduce lipid accumulation in the mouse liver, decreasing total hepatic triglyceride and cholesterol content compared to the vehicle group. gene analysis demonstrated that l-165041 lowered hepatic expression of pparγ, apolipoprotein b, il-1β, and interleukin-6. in contrast, l-165041 increased hepatic expressions of pparδ, lipoprotein lipase, and atp-binding cassette transporter g1 (abcg1) [2]. | | target | PPARδ | | 貯蔵 | Desiccate at RT | | 参考文献 | [1] wurch t, junquero d, delhon a, pauwels j. pharmacological analysis of wild-type alpha, gamma and delta subtypes of the human peroxisome proliferator-activated receptor. naunyn schmiedebergs arch pharmacol. 2002 feb;365(2):133-40.
[2] lim hj, park jh, lee s, choi he, lee ks, park hy. ppardelta ligand l-165041 ameliorates western diet-induced hepatic lipid accumulation and inflammation in ldlr-/- mice. eur j pharmacol. 2009 nov 10;622(1-3):45-51. |
|