|
ChemicalBook Optimization Suppliers |
|
| 融点 | 288-290°C | | 比重(密度) | 0.781 g/cm3 | | 貯蔵温度 | room temp | | 溶解性 | H2O: 50 mg/mL | | 外見 | White solid | | 酸解離定数(Pka) | 4(at 25℃) | | 色 | White to Almost white | | 水溶解度 | Soluble in water to 50mg/ml. | | Merck | 14,3081 | | BCS Class | 2 | | 安定性: | Stable. | | InChI | InChI=1S/C14H11Cl2NO2.Na/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19;/h1-7,17H,8H2,(H,18,19);/q;+1/p-1 | | InChIKey | KPHWPUGNDIVLNH-UHFFFAOYSA-M | | SMILES | C1(=CC=CC=C1CC([O-])=O)NC1=C(Cl)C=CC=C1Cl.[Na+] | | CAS データベース | 15307-79-6(CAS DataBase Reference) |
| | ジクロフェナクナトリウム Usage And Synthesis |
| 外観 | 白色〜わずかにうすい黄色, 結晶〜粉末 | | 溶解性 | メタノール,エタノールに易溶、水,酢酸にやや可溶、アセトンに難溶、エーテル,クロロホルムにほとんど不溶。 | | 用途 | フェニル酢酸系化合物です。シクロオキシゲナーゼ(COX)を阻害し、プロスタグランジン生合成抑制作用を示します。
| | 用途 | 薬理・生理作用研究用。 | | 効能 | 鎮痛薬, 抗炎症薬, 解熱薬, シクロオキシゲナーゼ阻害薬 | | 商品名 | ジクロード (わかもと製薬); ナボール (久光製薬); ナボール (久光製薬); ナボール (久光製薬); ナボール (久光製薬); ナボール (久光製薬); ボルタレン (ノバルティスファーマ); ボルタレン (ノバルティスファーマ); ボルタレン (同仁医薬化工); ボルタレン (同仁医薬化工); ボルタレン (同仁医薬化工); ボルタレン (同仁医薬化工) | | 説明 | Diclofenac is a non-steroidal anti-inflammatory drug (NSAID) and COX inhibitor (IC50s = 0.9-2.7 and 1.5-20 μM, for human COX-1 and COX-2, respectively). It is also an active metabolite of diclofenac methyl ester and diclofenac amide . Diclofenac inhibits release of arachidonic acid (Item Nos. 90010 | 90010.1 | 10006607) induced by A23187 in isolated rat peritoneal neutrophils and macrophages (IC50s = 60 and 10 μM, respectively). Transdermal administration of diclofenac inhibits carrageenan-induced paw edema in rats. Formulations containing diclofenac have been used in the treatment of pain associated with osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis. | | 化学的特性 | Off-White Crystalline Solid | | Originator | Voltaren,Fujisawa,Japan,1974 | | 使用 | Diclofenac sodium is a nonsteroidal anti-inflammatory compound and cyclooxygenase (COX) inhibitor. Oxidation of diclofenac sodium produces the metabolite 4'-hydroxy diclofenac) which demonstrates specific inhibition of Cox-2. Inhibition of Cox by diclofenac and 4'-hydroxy diclofenac suppresses prostaglandin E2 synthesis, producing anti-inflammatory and analgesic effects. Diclofenac is also shown to stabilize the native tetrameric conformation of transthyretin (TTR) fibrils, preventing the formation of insoluble amyloidogenic TTR deposits. Diclofenac Sodium is a substrate of CYP2C9. It is also used as an inhibitor of Cox-1 and Cox-2. | | 定義 | ChEBI: Diclofenac sodium is the sodium salt of diclofenac. It contains a diclofenac(1-). | | Manufacturing Process | Four grams of N-chloroacetyl-N-phenyl-2,6-dichloroaniline and 4 grams of
aluminum chloride are well mixed together and heated for 2 hours at 160°C.
The melt is cooled and poured onto about 50 grams of ice while it is still
warm. The oil which separates is dissolved in 50 ml of chloroform, the
chloroform solution is washed with 10 ml of water, dried over sodium sulfate
and concentrated under 11 torr. The residue is distilled. The 1-(2,6-
dichlorophenyl)-2-indolinone melts at 126°-127°C.
A solution of 186 grams of 1-(2,6-dichlorophenyl)-2-indolinone in 660 ml of
ethanol and 660 ml of 2 N sodium hydroxide solution is refluxed for 4 hours.
The solution is then cooled and left to stand for 4 hours at 0°-5°C. The
crystals which form are filtered off and recrystallized from water. The sodium
salt of 2-(2,6-dichloroanilino)-phenylacetic acid melts at 283°-285°C. The
yield is 97% of theoretical, according to US Patent 3,558,690. | | brand name | Solaraze (Bioglan); Voltaren (Novartis);Aflamin;Alfamin;Allvoran;Artren;Blesin;B-voltaren;Cgp 9194;Chlorgyl;Ct-diclo;Dichloronic;Diclo attritin;Diclo spondril;Diclo-attritin;Diclo-burg;Diclo-phlohont;Diclo-puren;Diclo-recip;Dicloreum;Diclo-spondyril;Diclo-wolf;Dolobasan;Dolotrem;Doragon;Duravolten;Duvavotten;Feloran;Fenoflam;Flogofenac;Flogogenac;Forgenac;Inflamac;Klast;Monoflam;Myogit;Neuro-effekton;Neurofenac;Neuro-voltaren;Neviodin;Olfen;Panamor;Rewodina;Rheumalgen;Rheumavincin-n;Seecoren;Shignol;Silino;Sofarin;Thicataren;Toryxil;Tsudomin;Voltarene. | | Therapeutic Function | Antiinflammatory | | 世界保健機関(WHO) | The World Health Organization currently has no information to
suggest that diclofenac is less safe than other widely available non-steroidal
antiinflammatory substances of this type, or that children are particularly liable to
react adversely. It is registered in many countries in several dosage forms,
including a 12.5 mg suppository indicated for juvenile arthritis. | | 一般的な説明 | Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Diclofenac is a derivative of benzeneacetic acid. It is categorized under the class of non-steroidal anti-inflammatory drugs (NSAIDs). It shows inflammatory, analgesic and antipyretic activities. | | Biochem/physiol Actions | Standard NSAID and cyclooxygenase (COX) inhibitor. Major metabolites are 4′-hydroxydiclofenac and 5′-hydroxydiclofenac. Has been used as substrate selective for CYP2C9. | | 臨床応用 | Diclofenac sodium is indicated for the treatment of rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis. | | 副作用 | The structure of diclofenac is a hybrid of a fenamate and an arylacetic acid. The compound is
used as its sodium salt for the symptomatic relief
of rheumatoid arthritis and osteoarthritis, including degenerative joint disease of the hip. The recommended dose is 75 – 150 mg/d which is clinically equivalent to 3.6g/d of aspirin. Gastrointestinal problems (ulceration and bleeding) and
adverse CNS reactions (dizziness and headache)
are the most commonly encountered adverse effects. | | 合成 | Synthesis: Acylation of N-phenyl-2,6- dichloroaniline with chloroacetyl chloride gives the corresponding chloroacetanilide, which is fused with aluminum chloride to give 1-(2,6- dichlorophenyl)-2-indolinone. Hydrolysis of the indolinone with dilute aqueous-alcoholic sodium hydroxide affords the desired sodium salt directly.
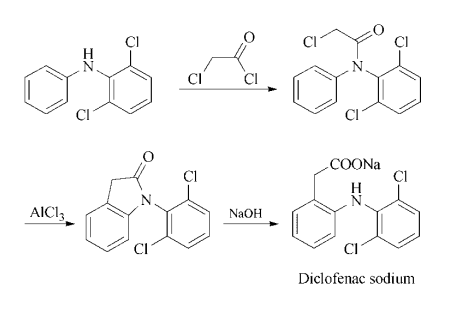 | | Veterinary Drugs and Treatments | The equine topical cream (Surpass?) is labeled for the control of
pain and inflammation associated with osteoarthritis in tarsal, carpal,
metacarpophalangeal, metarsophalangeal, and proximal interphalangeal
(hock, knee, fetlock, pastern) joints for use up to 10 days
duration. While, theoretically, diclofenac could be used systemically
(orally) in other veterinary species, there are approved and safer
alternatives. | | 薬物相互作用 | Potentially hazardous interactions with other drugs
antagonism of hypotensive effect; increased risk of
nephrotoxicity and hyperkalaemia.
Analgesics: avoid concomitant use of 2 or more
NSAIDs, including aspirin (increased side effects);
avoid with ketorolac (increased risk of side effects
and haemorrhage).
Antibacterials: possibly increased risk of convulsions
with quinolones; concentration reduced by
rifampicin.
Anticoagulants: effects of coumarins and
phenindione enhanced; possibly increased risk of
bleeding with heparins, dabigatran and edoxaban -
avoid long term use with edoxaban; increased risk of
haemorrhage with IV diclofenac - avoid.
Antidepressants: increased risk of bleeding with
SSRIs and venlaflaxine.
Antidiabetic agents: effects of sulphonylureas
enhanced.
Antiepileptics: possibly increased phenytoin
concentration.
Ciclosporin: may potentiate nephrotoxicity;
concentration increased by ciclosporin.
Cytotoxics: reduced excretion of methotrexate;
increased risk of bleeding with erlotinib. | | 代謝 | Diclofenac undergoes first-pass metabolism and is It is then excreted in the form of glucuronide and sulfate
conjugates, mainly in the urine (about 60%) but also in
the bile (about 35%).
then extensively metabolised to 4′-hydroxydiclofenac,
5-hydroxydiclofenac, 3′-hydroxydiclofenac, and
4′,5-dihydroxydiclofenac by glucuronidation of the intact
molecule or more commonly by single and multiple
hydroxylation followed by glucuronidation. | | 貯蔵 | Store at RT |
|