|
ChemicalBook Optimization Suppliers |
| 名前: |
BEST-REAGENT |
| 電話番号: |
400-1166-196 18981987031 |
| 電子メール: |
cdhxsj@163.com |
|
| 化学名: | カナマイシン硫酸塩 | | 英語化学名: | KANAMYCIN SULFATE | | 别名: | 2-(Aminomethyl)-6-[4,6-diamino-3-[4-amino-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-2-hydroxycyclohexyl]oxyoxane-3,4,5-triol sulfate;C18H36-37N4-5O10-11H2SO4;Kanmycin MonosulfateSulfate;kanamycin sulfate mixture of components A;KANAMYCIN SULFATE MIXTURE OF COMPONENTS A,B AND C;KANAMYCIN MONOSULFATE;KANAMYCIN MONOSULFATE SALT;KANAMYCIN MONO SULPHATE | | CAS番号: | 70560-51-9 | | 分子式: | C18H38N4O15S | | 分子量: | 582.58 | | EINECS: | 684-844-0 | | カテゴリ情報: | | | Mol File: | 70560-51-9.mol | 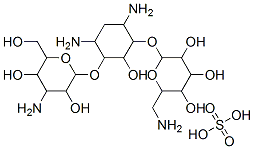 |
| 貯蔵温度 | 2-8°C | | 溶解性 | H2O: 10-50 mg/mL As a stock solution. Stock solutions should be stored at 2-8°C. Stable at 37°C for 5 days. | | 外見 | powder | | 色 | white to off-white | | BRN | 3874279 | | CAS データベース | 70560-51-9(CAS DataBase Reference) |
| | カナマイシン硫酸塩 Usage And Synthesis |
| Originator | Kantrex,Bristol,US,1958 | | 使用 | Kanamycin Sulfate is an aminoglycoside-antibiotic with broad antibacterial spectrum. | | Manufacturing Process | As described in US Patent 2,931,798, Streptomyces kanamyceticus (K2-J) was
first cultured in shake flasks in the following media: (a) 0.75% meat extract,
0.75% peptone, 0.3% NaCl, with 1.0% of starch, dextrin, maltose, glucose,
lactose, sucrose or glycerol; or (b) 2.0% soybean meal, 0.05% KCl, 0.05%
MgSO4 · 7H2O, 0.5% NaCl, 0.2% NaNO3, with 1.0% of starch, dextrin,
maltose, glucose, lactose, sucrose or glycerol. The initial pH of all media was
adjusted to 7.0. After 24 to 48 hours shaking in some cases the pH decreased
to about 6.0 to 6.8, but from 72 to 120 hours the pH rose and became 7.5 to
8.6. The production of kanamycin was apparent after 48 hours and, depending
on the media; the maximum production was found after 72 to 120 hours.
The yield was highest with starch or dextrin, intermediate and about the same
with sucrose, glucose, maltose and lactose and poorest with glycerol.
Kanamycin was produced by media containing soybean meal, peanut meal,
cottonseed meal, corn steep liquor, peptone, yeast extract or meat extract,
with or without sodium nitrate. Commercially available soybean meal was
recognized to be one of the best nitrogen sources. The addition of corn steep
liquor, peptone, yeast extract or nitrate to the soybean meal promoted the
production of kanamycin.
The brownish white kanamycin (5 g) was dissolved in 50 ml of 60% aqueous
methanol, insoluble material was removed and to the filtrate 40 ml of 60%
aqueous methanol containing 2,000 mg of ammonium sulfate was added, and
the precipitated kanamycin sulfate was collected, washed with 50 ml of 80%
aqueous methanol, and dried. Thus, 4.5 g of kanamycin sulfate was obtained
as a light brownish powder. | | Therapeutic Function | Antibacterial | | 臨床応用 | Kanamycin Sulfate was isolated in 1957 by Umezawaand coworkers from Streptomyces kanamyceticus. Its activityagainst mycobacteria and many intestinal bacteria, aswell as several pathogens that show resistance to other antibiotics,brought a great deal of attention to this antibiotic.As a result, kanamycin was tested and released for medicaluse in a very short time.
The use of kanamycin in the United States usually is restrictedto infections of the intestinal tract (e.g., bacillarydysentery) and to systemic infections arising from Gramnegativebacilli (e.g., Klebsiella, Proteus, Enterobacter, andSerratia spp.) that have developed resistance to other antibiotics.It has also been recommended for preoperative antisepsisof the bowel. It is absorbed poorly from the intestinaltract; consequently, systemic infections must be treated byintramuscular or (for serious infections) intravenous injections.These injections are rather painful, and the concomitantuse of a local anesthetic is indicated. The use ofkanamycin in the treatment of TB has not been widely advocatedsince the discovery that mycobacteria develop resistancevery rapidly. In fact, both clinical experience andexperimental work indicate that kanamycin developscross-resistance in the tubercle bacilli with dihydrostreptomycin,viomycin, and other antitubercular drugs. Like streptomycin,kanamycin may cause decreased or complete lossof hearing. On development of such symptoms, its useshould be stopped immediately. |
|