|
ChemicalBook Optimization Suppliers |
|
| 化学名: | エキセメスタン | | 英語化学名: | Exemestane | | 别名: | ExeMestane (USP);1,4-Androstadien-3,17-dione-6-Methylene-d2;(8R,9S,10R,13S,14S)-10,13-Dimethyl-6-methylene-7,8,9,11,12,13,15,16-octahydro-6H-cyclopenta[a]phenanthrene-3,17(10H,14H)-dione;ExeMestine;(8R,9S,10R,13S,14S)-10,13-diMethyl-6-Methylene-7,8,9,10,11,12,13,14,15,16-decahydro-3H-cyclopenta[a]phenanthrene-3,17(6H)-dione;ExeMestane SynonyMs 6-Methylenandrosta-1,4-diene-3,17-dione;PNU155971;Exemestane for system suitability | | CAS番号: | 107868-30-4 | | 分子式: | C20H24O2 | | 分子量: | 296.4 | | EINECS: | 643-090-2 | | カテゴリ情報: | Intermediates & Fine Chemicals;Pharmaceuticals;Steroids;Pfizer compounds;Steroid and Hormone;API;Inhibitors;Anti-cancer&immunity;Active Pharmaceutical Ingredients;chiral;Antineoplastic (Hormonal);107868-30-4 | | Mol File: | 107868-30-4.mol | 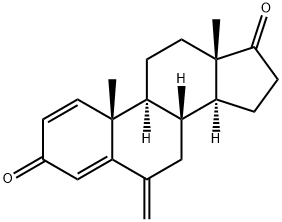 |
| 融点 | 155.13°C | | 沸点 | 453.7±45.0 °C(Predicted) | | 比重(密度) | 1.13±0.1 g/cm3(Predicted) | | 貯蔵温度 | 2-8°C | | 溶解性 | DMSO: ≥20mg/mL | | 外見 | powder | | 色 | white to off-white | | 光学活性 (optical activity) | [α]/D +250 to +300°, c = 1 in methanol | | InChI | InChI=1S/C20H24O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h6,8,11,14-16H,1,4-5,7,9-10H2,2-3H3/t14-,15-,16-,19+,20-/m0/s1 | | InChIKey | BFYIZQONLCFLEV-DAELLWKTSA-N | | SMILES | C1(=O)C=C2[C@](C)(C=C1)[C@]1([H])[C@]([H])([C@@]3([H])[C@@](CC1)(C)C(=O)CC3)CC2=C | | CAS データベース | 107868-30-4(CAS DataBase Reference) |
| | エキセメスタン Usage And Synthesis |
| 外観 | 白色~うすい黄色, 結晶性粉末~粉末 | | 用途 | ステロイド性アロマターゼ不可逆的阻害剤です(IC50 = 20 nmol/l)。
アロマターゼを不安定化させ、エストロゲンレベルを減少させます。経口活性があります。 | | 用途 | エキセメスタンは、第3世代アロマターゼ阻害薬のひとつ。乳癌の治療に用いられる。
レトロゾールまたはアナストロゾールに抵抗性の局所進行性または転移性の閉経後乳癌にエキセメスタンとエベロリムスの併用は有用であった。
| | 用途 | 非可逆的アロマターゼ阻害作
用を示します | | 効能 | 抗悪性腫瘍薬, エストロゲン生合成阻害薬 | | 商品名 | アロマシン (ファイザー) | | 説明 | Exemestane was launched in US and other countries for the treatment of estrogendependent
tumors and postmenopausal breast cancer. It is a novel steroidal compound
structurally related to the natural substrate for the biosynthesis of estrogen,
androstanedione, and can be synthesized by methylidenation of androsta-1, 4-dien-
17beta-ol-3-one in 6 position then oxidation of the alcohol function. Exemestane is an
irreversible inactivator of the aromatase enzyme system, so inducing in vivo a dose-related
sustained suppression of serum estrogen and minimal endocrine activity. It is the first
steroidal representative of the third-generation of orally active aromatase inhibitors with a
highly potent and selective mechanism of action, displaying good tolerability and safety
profile. In rats with DMBA-induced mammary tumors, 10 to 100 mglkg of exemestan
administered po twice-daily for 4 weeks resulted in 76 to 88% regression. In women failing
anti-estrogen therapy with tamoxifen, this agent has demonstrated high activity in locally
advanced or metastatic disease. In addition, it may also have potential for breast cancer
prevention. | | 化学的特性 | white to light yellow crystal powder | | Originator | Farmitalia Carlo Erba (Italy) | | 使用 | antiinfective | | 使用 | An antineoplastic (hormonal) | | 使用 | Labeled Exemestane, intended for use as an internal standard for the quantification of Exemestane by GC- or LC-mass spectrometry. | | 定義 | ChEBI: A 17-oxo steroid that is androsta-1,4-diene-3,17-dione in which the hydrogens at position 6 are replaced by a double bond to a methylene group. A selective inhibitor of the aromatase (oestrogen synthase) system, it is used in the treatment of advanced brea
t cancer. | | Manufacturing Process | 0.50 g of 6-methylenandrost-4-ene-3,17-dione and 0.57 g of
dichlorodicyanobenzoquinone were refluxed in 20 ml of anhydrous dioxane for
about 15 hours. To remove the DDQ the suspension was filtered through
alumina. After evaporation of the solvent the residue was dissolved in ethyl
acetate, the organic layer washed with water, dried over sodium sulfate and
the solvent removed under vacuum. The crude product was chromatographed
on silica gel using hexane/ethyl acetate to yield 0.25 g of pure 6-
methylenandrosta-1,4-diene-3,17-dione, m.p. 188-191°C, λmax 247 nm (ε
13.750). | | brand name | Aromasin (Pharmacia& Upjohn). | | Therapeutic Function | Antineoplastic | | 一般的な説明 | Exemestane, 6-methylenandrosta-1,4-diene-3,17-dione (Aromasin), is the first steroid-basedaromatase inhibitor approved for the treatment of breastcancer in the United States. It is a mechanism-based inactivatorthat irreversibly inhibits the enzyme. Plasma estrogenlevels are reduced by 85% to 95% within 2 to 3 days, and effectslast 4 to 5 days. Exemestane does not inhibit any of themajor cytochromes P450 and has essentially no interactionwith steroid receptors, with only a very weak affinity for theAR. The 17β-hydroxyexemestane reduction product, however,has much higher affinity for the AR than the parent(still several fold less than DHT, 0.28% for parent vs. 30%for metabolite). The clinical significance of the affinity islikely minimal because of the low levels of the metaboliteproduced. | | 危険性 | A reproductive hazard. | | Biochem/physiol Actions | Exemestane is a steroidal antiestrogen and irreversible aromatase inhibitor. Exemestane acts as a false substrate for the aromatase enzyme. Exemestane also prevents the conversion of androgens to estrogens and is used to treat estrogen-dependent breast cancer. | | 臨床応用 | Irreversible, steroidal aromatase inhibitor:Treatment of breast cancer | | 代謝 | Metabolised via oxidation by the cytochrome
P450 isoenzyme CYP3A4, and via reduction by
aldoketoreductase. Metabolites are excreted in the urine
(39-45%) and faeces (36-48%). | | 参考文献 | [1] franco buzzetti, enrico di salle, antonio longo, gabriella briatico. synthesis and aromatase inhibition by potential metabolites of exemestane (6-methylenandrosta-1,4-diene-3,17-dione). steroids. november 1993. 58(11): 527-532.
[2] gustavo de albuquerque cavalcanti, bruno carius garrido, felipe dias leal, monica costa padilha, xavier de la torre, francisco radler de aquino neto. detection of new urinary exemestane metabolites by gas chromatography coupled to mass spectrometry. steroids. september–october 2011. 76(10-11): 1010-1015.
[3] stephanie a. jones, stephen e. jones. exemestane: a novel aromatase inactivator for breast cancer. clinical breast cancer. october 2000. 1(3): 211-216. |
|