|
ChemicalBook Optimization Suppliers |
|
| 化学名: | アルチカイン塩酸塩 | | 英語化学名: | Articaine hydrochloride | | 别名: | methyl 4-methyl-3-[[(1-oxo-2-(propylamino)propyl]amino]-2-thenoate monohydrochloride;CarticaineHCl;CARTICAINE HYDROCHLORIDE;Articaine hydrochloride;Methyl 4-methyl-3-(2-propylaminopropanoylamino)thiophene-2-carboxylate hydrochloride;Articaine Hydrochloride (125 mg);4-methyl-3-[2-(propylamino)propanoylamino]thiophene-2-carboxylic acid methyl ester hydrochloride;Methyl 4-methyl-3-[(N-propylalanyl)amino]-2-thiophenecarboxylate hydrochloride | | CAS番号: | 23964-57-0 | | 分子式: | C13H20N2O3S.ClH | | 分子量: | 320.839 | | EINECS: | 245-957-7 | | カテゴリ情報: | research chemical;Pharmaceutical intermediate;local anesthetic;API;BDO | | Mol File: | 23964-57-0.mol | 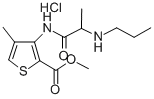 |
| 融点 | 177-178° | | 貯蔵温度 | Keep in dark place,Inert atmosphere,2-8°C | | 溶解性 | H2O: soluble20mg/mL, clear | | 外見 | powder | | 色 | white to beige | | 安定性: | Hygroscopic | | InChI | InChI=1S/C13H20N2O3S.ClH/c1-5-6-14-9(3)12(16)15-10-8(2)7-19-11(10)13(17)18-4;/h7,9,14H,5-6H2,1-4H3,(H,15,16);1H | | InChIKey | GDWDBGSWGNEMGJ-UHFFFAOYSA-N | | SMILES | C1(NC(=O)C(C)NCCC)C(C)=CSC=1C(=O)OC.Cl |
| 主な危険性 | Xi | | Rフレーズ | 36/37/38 | | Sフレーズ | 26 | | WGK Germany | 3 | | 国連危険物分類 | IRRITANT | | HSコード | 2934990002 | | 毒性 | LD50 i.v. in mice: 37 mg/kg (Muschaweck, Rippel) |
| | アルチカイン塩酸塩 Usage And Synthesis |
| 効能 | 局所麻酔薬 | | 説明 | Articaine hydrochloride is a thiophene-containing local anesthetic pharmacologically similar to mepivacaine. Most local anesthetics act by abolishing voltage-gated sodium channel currents indiscriminately in all populations of neurons. Articaine is an amide local anesthetic most commonly used in dentistry. It possesses a unique ester side chain that is metabolized rapidly by circulating esterases. Articaine is reported to modify cardiac action potentials and ion currents only at concentrations higher than the therapeutic range needed to inhibit voltage-gated sodium channels. | | 化学的特性 | White or almost white, crystalline powder. | | Originator | Ultracain,Hoechst,W. Germany,1976 | | 使用 | Articaine hydrochloride, belonging to the group of amide anesthetics, is a local anesthetic with a short duration of action. It is utilized for regional anesthesia in day-case surgeries such as arthroscopy, hand and foot surgery, and dentistry. | | Manufacturing Process | 3-α-Chloropropionylamino-2-carbomethoxy-4-methylthiophene (prepared from
3-amino-2-carbomethoxy-4-methylthiophene and chloropropionyl chloride)
was dissolved in toluene and n-propylamine added. The whole mixture was
heated to boiling for 6 to 7 hours. After cooling, the propylamine
hydrochloride that had formed was removed by washing with water, The
toluene phase was dried with sodium sulfate, and then the solvent and excess
propylamine were removed by distillation. The oily residue was taken up in
ether. The hydrochloride of 3-n-propylamino-α-propionylamino-2-
carbomethoxy-4-methylthiophene was obtained by introducing hydrogen
chloride gas or by means of methanolic hydrogen chloride. The base boils at
162°C to 167°C under 0.3 mm of mercury pressure and the hydrochloride
melts at 177°C to 178°C. | | brand name | Septanest (Cilag-Chemie, Switzerland); Septocaine
(Cilag-Chemie, Switzerland); Ultracaine (Hoechst
AG, Germany). | | Therapeutic Function | Local anesthetic | | 接触アレルゲン | This local amide-type anesthetic is seldom reported as
allergenic even in patients sensitized to other amidetype
molecules like lidocaine, prilocaine, mepivacaine,
or bupivacaine. | | Biochem/physiol Actions | Articaine hydrochloride helps to alleviate pain and blocks transmission of the pain signal. Articaine is an amide local anesthetic widely used in dentistry. Articaine acts by inhibition of nerve impulse conduction by blockade of sodium channels. | | 代謝 | Articaine is the only amide local anesthetic containing a thiophene aromatic ring rather than benzene. Because articaine hydrochloride is the only widely used amide local anesthetic that also contains an ester group, biotransformation of articaine hydrochloride occurs in both plasma (hydrolysis by plasma esterase—similarly to other ester local anesthetics) and liver (hepatic microsomal enzymes—similarly to other amide local anesthetics). The metabolism of articaine hydrochloride is initiated by hydrolysis of the carboxylic acid ester groups to obtain free carboxylic acid. Its primary metabolite, articainic acid, is pharmacologically inactive, undergoing additional biotransformation to form articainic acid glucuronide. Additional metabolites have been detected in animal studies. From this point, the reaction can follow several pathways: cleavage of carboxylic acid, formation of an acid amino group by internal cyclization, and oxidation. | | Mode of action | Articaine Hydrochloride is the hydrochloride salt form of articaine, an amide-type local anesthetic. Its mechanism of action involves reversible blockage of nerve impulse conduction through binding to specific sodium ion channels on the membrane, thereby interfering with the electrical excitation in the nerve, slowing the propagation of the nerve impulse and reducing the rate of action potential rise. This results in a loss of sensation at the site of injection. Articaine hydrochloride is used for relief of pain in minor operations, usually in combination with the vasoconstrictor epinephrine. |
|