|
ChemicalBook Optimization Suppliers |
| 名前: |
LGM Pharma |
| 電話番号: |
1-(800)-881-8210 |
| 電子メール: |
inquiries@lgmpharma.com |
|
| 化学名: | ペミロラスト | | 英語化学名: | PEMIROLAST | | 别名: | PEMIROLAST;Pemiroplastpotassium;9-Methyl-3-(1H-tetrazol-5-y1)-4H-pyrido[1,2-α]pyrimidin-4-one;Pemilaston;9-Methyl-3-(1H-tetrazol-5-yl)-4H-pyrido[1,2-a]pyrimidin-4-one;100299-08-9 (Potassium salt);4H-Pyrido(1,2-A)pyrimidin-4-one, 9-methyl-3-(1H-tetrazol-5-yl)-;Hsdb 7291 | | CAS番号: | 69372-19-6 | | 分子式: | C10H8N6O | | 分子量: | 228.21 | | EINECS: | | | カテゴリ情報: | | | Mol File: | 69372-19-6.mol | 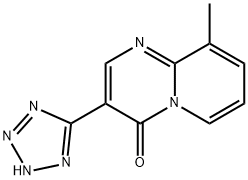 |
| | ペミロラスト Usage And Synthesis |
| 効能 | 抗アレルギー薬, メディエーター遊離抑制薬 | | Originator | Alamast, Santen | | 使用 | Anti-allergic;
inhibitor (mediator release). | | 定義 | ChEBI: Pemirolast is a pyridopyrimidine. | | Manufacturing Process | Ferrous nitrate hexahydrate (60 mg) followed by sodium (4.5 g, 0.196 gatom) were added to liquid ammonia. To this mixture was added a solution of 3-methylpyridine (10.0 g, 0.093 mole) in N,N-dimethylaniline (21 ml) over a period of 5 min. The ammonia was allowed to evaporate and the residue heated under nitrogen by means of an oil bath maintained at 180°C for 18 h. The cooled residue was treated with ice (50 g) followed by 2 N sodium hydroxide (50 ml). The mixture was triturated for 2 h and then filtered. The collected solid was washed with boiling toluene (2 times 100 ml). The toluene layer was separated from the combined filtrate and washings, concentrated to about 50 ml and extracted with 5% aqueous acetic acid (5 times 20 ml). The combined extracts were filtered and reduced to dryness. The residue was recrystallized from methylcyclohexane to give 2-amino-3-methylpyridine acetate (4.9 g, 29%), melting point 85°-95°C. The acetate (2.5 g, 1.37 mmoles) was briefly suspended in 1 N sodium hydroxide (50 ml). The mixture was extracted with methylene chloride. The extract was washed with water, dried, and concentrated to give 2-amino-3-methylpyridine as an oil.
A solution of 2-amino-3-methylpyridine (5.0 g, 0.0462 mole) and ethyl ethoxymethylenecyanoacetate (7.82 g, 0.0462 mole) in toluene (4 ml) was heated for 15 min by means of an oil bath maintained at 100°C. The solution was cooled and the crude product (9.1 g, 85%) collected by filtration. The product was recrystallized from 2-propanol to give an analytical sample of ethyl 2-cyano-3-(3-methyl-2-pyridylamino)acrylate, melting point 144°146°C.
Aluminum chloride (3.51 g, 0.0263 mole) was added to cold (-30°C) tetrahydrofuran (180 ml). Sodium azide (5.12 g, 0.0788 mole) was added and the mixture heated under reflux for 30 min. The mixture was cooled to 5°C. Ethyl 2-cyano-3-(3-methyl-2-pyridylamino)acrylate (5.0 g, 0.0216 mole) was added and the mixture heated under reflux for 18 h. The tetrahydrofuran was removed under reduced pressure. The residue was treated with ice water (100 ml) and acidified to pH 3 with 6 N hydrochloric acid. The mixture was filtered and the collected solid recrystallized from N,N-dimethylformamide to give the 9-methyl-3-(1H-tetrazol-5-yl)-4H-pyrido[1,2-a]pyrimidin-4-one (2.5 g, 50.7%). Melting point 310°-311°C, dec.
Potassium hydroxide was added dropwise to a stirred mixture of 9-methyl-3(1H-tetrazol-5-yl)-4H-pyrido[1,2-a]pyrimidin-4-one in water .The mixture was diluted with water to a volume of about 300 ml and was then heated to a temperature of 50°C during 2 min. The mixture was filtered and the water removed from the filtrate by lyophilization. The residue was recrystallized from water:ethanol to give the 9-methyl-3-(1H-tetrazol-5-yl)-4H-pyrido[1,2
2632 a]pyrimidin-4-one potassium salt | | brand name | Alamast (Sanofi Winthrop). | | Therapeutic Function | Antiallergic, Antiulcer | | 臨床応用 | Pemirolast, with an acidic tetrazole isosteric replacement for a carboxylic acid functionality, is
used topically in the eye to prevent itching associated with allergic conjunctivitis. It is an
inhibitor of the release of histamine and other inflammatory mediators, including leukotrienes.
Significant use as a systemic agent has been reported, and it has been shown to be of value
in preventing restenosis after percutaneous coronary angiopathy. |
|