|
ChemicalBook Optimization Suppliers |
|
| 化学名: | イソトレチノイン | | 英語化学名: | Isotretinoin | | 别名: | Isotretinoin-D5/13-cis-Retinoic acid-D5;Roaccutan;(2Z,4E,6E,8E)-3,7-diMethyl-9-(2,6,6-triMethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraenoic acid;A different diMension acid;Isotretinoin API;(2Z,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic Acid
Isotretinoin;Isotretinoin (Roaccutane);Isotretinoin (EP5.0) | | CAS番号: | 4759-48-2 | | 分子式: | C20H28O2 | | 分子量: | 300.44 | | EINECS: | 225-296-0 | | カテゴリ情報: | Other APIs;intermediates;BUSPAR;API;Organic acids;APIs;Intermediates & Fine Chemicals;Pharmaceuticals;Retinoids;Antibiotics;4759-48-2 | | Mol File: | 4759-48-2.mol | 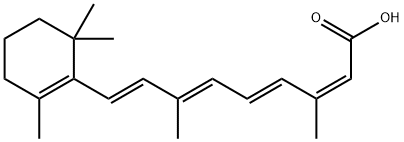 |
| 融点 | 172-175 °C (lit.) | | 沸点 | 381.66°C (rough estimate) | | 比重(密度) | 1.0597 (rough estimate) | | 屈折率 | 1.4800 (estimate) | | 貯蔵温度 | -20°C | | 溶解性 | Practically insoluble in water, soluble in methylene chloride, slightly soluble in ethanol (96 per cent). It is sensitive to air, heat and light, especially in solution. Carry out all operations as rapidly as possible and avoid exposure to actinic light; use freshly prepared solutions. | | 酸解離定数(Pka) | 4.76±0.33(Predicted) | | 外見 | Fine Crystalline Powder | | 色 | Yellow-orange to orange | | 水溶解度 | insoluble | | 極大吸収波長 (λmax) | 354nm(EtOH)(lit.) | | Merck | 14,5228 | | 安定性: | Stable, but probably air and light sensitive. Combustible. Incompatible with strong oxidizing agents. | | InChIKey | SHGAZHPCJJPHSC-YCNIQYBTSA-N | | CAS データベース | 4759-48-2(CAS DataBase Reference) | | EPAの化学物質情報 | Isotretinoin (4759-48-2) |
| | イソトレチノイン Usage And Synthesis |
| 外観 | 黄色~褐色, 結晶性粉末~粉末 | | 溶解性 | アセトン(0.005+1) 全溶エタノール(0.005+1) 全溶メタノール(0.005+1) 全溶水(0.005+1) 不溶エタノール及びアセトンに溶け、水にほとんど溶けない。 | | 用途 | 薬理・生理作用研究用。 | | 用途 | 国際一般名でイソトレチノイン(英: isotretinoin)は、13-シスレチノイン酸(英: 13-cis-Retinoic Acid)とも呼ばれ、主に尋常性痤瘡(ニキビ)の治療で使用される。 | | 効能 | 抗ニキビ薬, 角質溶解薬, レチノイン酸受容体(RAR)作動薬 | | 使用上の注意 | 安定性が悪いので一回使用量に小分けし、不活性ガスを充填しておく事が望ましい。また、本品は光によって異性化する性質がある。不活性ガス封入 | | 説明 | Isotretinoin is the 9-cis isomer of retinoic acid, a close relative of retinol, or vitamin A. It was originally developed to treat cystic acne, and today this is still its primary use despite several more modern applications of the drug, including a treatment for pancreatic and brain cancers. First shown to be an effective treatment for acne in 1982, its development stemmed from advances in knowledge of the effects of vitamin A to reduce or eliminate sebum production. Since that time, however, several instances of deleterious effects became well known, most notably birth defects arising from the use of isotretinoin. | | 化学的特性 | Yellow or orange crystalline powder or crystal, insoluble in water, slightly soluble in ethanol, very slightly soluble in ether, soluble in chloroform. | | Originator | Accutane,Roche | | 使用 | isotretinoin is a retinoid derivative with improved bioavailability and percutaneous absorption for acne treatment products. Presently being studied in conjuction with the treatment of photoaged skin. | | 定義 | ChEBI: Isotretinoin is a retinoic acid that is all-trans-retinoic acid in which the double bond which is alpha,beta- to the carboxy group is isomerised to Z configuration. A synthetic retinoid, it is used for the treatment of severe cases of acne and other skin diseases. It has a role as a keratolytic drug, an antineoplastic agent and a teratogenic agent. It is a conjugate acid of a 13-cis-retinoate. | | 適応症 | Isotretinoin (Accutane) alters keratinization in the
acroinfundibulum of sebaceous glands and shrinks
them, thereby reducing sebum excretion and comedogenesis.
These features underlie its usefulness in acne
vulgaris, since sebum secretion is a hallmark of acneprone
skin. Furthermore, the drug has antiinflammatory
activity. | | 製造方法 | Preparation of isotretinoin in a single step from β-ionone and ethyl chloride are first reacted together after which the product is further reacted with triphenylphosphine to obtain Triphenyl salt. The Triphenyl salt is further reacted with cyclopentenone derivative to produce isotretinoin and its 8Z isomer. separate out the 8Z isomer and convert it to isotretinoin through isomerization with the help of nitric acid. | | brand name | Accutane (Roche); Amnesteem
(Genpharm); Claravis (Barr); Sotret (Ranbaxy);Accutane roche;Apsor;Isotretinoin;Neovamin a acid;Neovitamin a acid;Ro 4-3780;Roacutan. | | Therapeutic Function | Antiacne, Keratolytic | | 世界保健機関(WHO) | Isotretinoin, a retinol derivative, was introduced in 1982 exclusively for the treatment of severe acne. Its use in pregnant women has
resulted in major fetal abnormalities. The manufacturer's information emphasizes
that the drug is teratogenic and must not be given to women who are pregnant,
and that contraceptive measures must be maintained for at least four weeks after
discontinuation of treatment. In some countries, blood banks are advised not to
accept as donors persons who have taken isotretinoin within the previous four
weeks. See also under retinol (vitamin A). | | 一般的な説明 | Yellow-orange to orange crystalline powder; orange-brown chunky solid. | | 空気と水の反応 | Insoluble in water. | | 反応プロフィール | An organic acid and unsaturated aliphatic hydrocarbon. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. | | 火災危険 | Flash point data for Isotretinoin are not available; however, Isotretinoin is probably combustible. | | Biochem/physiol Actions | 13-cis-Retinoic acid (RA) has anti-inflammatory and anti-tumor action. The action of RA is mediated through RAR-β and RAR-α receptors. RA attenuates iNOS expression and activity in cytokine-stimulated murine mesangial cells. It induces mitochondrial membrane permeability transition, observed as swelling and as a decrease in membrane potential, and stimulates the release of cytochrome c implicating mechanisms through the apoptosis pathway. These activities are reversed by EGTA and cyclosporin A. RA also increases MMP-1 protein expression partially via increased transcription. | | 作用機序 | Isotretinoin binds to and activates nuclear retinoic acid receptors (RARs); activated RARs serve as transcription factors that promote cell differentiation and apoptosis. This agent also exhibits immunomodulatory and anti-inflammatory responses and inhibits ornithine decarboxylase, thereby decreasing polyamine synthesis and keratinization. Isotretinoin is rapidly absorbed orally, with peak blood concentrations 3 hours after ingestion. It is not stored in tissue, and the elimination half-life is 10 to 20 hours, either after a single dose or during chronic therapy. | | 臨床応用 | Isotretinoin is most useful for the treatment of severe
recalcitrant nodular acne vulgaris. It may also be helpful in other disorders of keratinization, but it is
not useful for psoriasis. High doses of isotretinoin
(2mg/kg/day) are effective as cancer chemoprevention
agents to reduce the frequency of cutaneous malignancies
in patients at increased risk, such as those with xeroderma
pigmentosum, an inherited disorder in which
DNA repair is deficient, or in immunosuppressed patients. | | 副作用 | Isotretinoin is teratogenic to humans and should not be administered to pregnant women or women contemplating pregnancy. Concomitant use of isotretinoin with drugs of the tetracycline class increases the incidence of Pseudotumor cerebri. There have been recent reports of an increased risk of depression, suicide, and suicide attempts in individuals taking isotretinoin, but the causality has not been absolutely proved.
Isotretinoin, like many retinoids, can lead to increase in serum aminotransferase levels, but, unlike acitretin and etretinate, isotretinoin has not been clearly implicated in cases of clinically apparent acute liver injury with jaundice. | | 安全性プロファイル | Poison by
intraperitoneal route. Moderately toxic by
ingestion. A human teratogen by ingestion
with fetal developmental abnormalities of
the skin and appendages and other postnatal
effects. Human reproductive effects. Human
systemic effects: decreased immune
response, darrhea, hypermouhty, irritative
dermatitis, sweating. Human mutation data
reported. An experimental teratogen. Other
experimental reproductive effects. When
heated to decomposition it emits acrid
smoke and irritating fumes. | | Veterinary Drugs and Treatments | Isotretinoin may be useful in treating a variety of dermatologicrelated
conditions,
including canine lamellar ichthyosis, cutaneus
T-cell lymphoma, intracutaneous cornifying epitheliomas,
multiple
epidermal inclusion cysts, comedo syndrome in Schnauzers,
and sebaceous adenitis seen in standard poodles.
Because of the concerns of teratogenic effects in humans, availability
to veterinarians may be restricted by the manufacturers and
drug distributors; obtaining the medication for veterinary patients
may be difficult. | | 環境運命予測 | In primates (including humans), isotretinoin (Accutane) is
metabolized to a more active form, 13-cis-4-oxo-retinoic acid,
which is able to move through the placental membrane. On
its own, however, Accutane (isotretinoin) is not particularly
motile across the placental barrier, and perhaps most interestingly
tends not to bind to cellular retinoid-binding proteins
or nuclear receptors. The rapid isomerization to the all-trans
isomer, the oxidation of Accutane (isotretinoin) to 13-cis-4-
oxo-retinoic acid, and the relatively high circulation times of
these compounds may be important in explaining the teratogenic
toxicity of Accutane (isotretinoin).
Some studies have more fully explored the metabolic
products of isotretinoin. For example, isotretinoin can be
metabolized in the liver by the cytochrome P450 microsomal
enzyme system – more specifically the CYP2C8, CYP2C,
CYP3A4, and CYP2B6 isoenzymes. The metabolites produced
are numerous, including retinoic acid (tretinoin), 4-oxo-isotretinoin,
and 4-oxo-retinoic acid (4-oxo-tretinoin). This relatively
large array of retinoid metabolites may produce a variety
of effects, most notably due to their higher potency as retinoids
compared to the parent compound (isotretinoin).
It is possible that these additional metabolites are capable
of binding to a variety of retinoid receptors in order to alter
gene expression and further transcription or transrepression in
protein synthesis, which may be responsible for the toxic effects
of isotretinoin. | | Toxicity evaluation | Direct studies focused on the environmental fate of Accutane
(isotretinoin) are rare in the literature. The pure compound is
insoluble in water, and highly lipophilic. Powders do not
aerosolize readily, and volatilization is extremely low. Isotretinoin
released into the environment would not be expected
to have high mobility in water or soil, and will most likely
become deposited in organic materials. Bioaccumulation is
possible, but isotretinoin is readily oxidized to form other
retinoids or metabolites that are expected to be mitigated via
natural biological pathways. |
|