|
ChemicalBook Optimization Suppliers |
| 名前: |
Wuhan Topule Gold |
| 電話番号: |
+86-02787215551 +86-19945035818 |
| 電子メール: |
2936752263@qq.com |
|
| 化学名: | ベネトクラクス | | 英語化学名: | ABT-199 | | 别名: | ABT-199;ABT-199 (GDC-0199);GDC-0199;ABT-199 100MG;2-(1H-Pyrrolo[2,3-b]pyridin-5-yloxy)-4-(4-((2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-enyl)met;ABT-199, Venetoclax;GDC 0199;GDC0199;Benzamide, 4-[4-[[2-(4-chlorophenyl)-4,4-dimethyl-1-cyclohexen-1-yl]methyl]-1-piperazinyl]-N-[[3-nitro-4-[[(tetrahydro-2H-pyran-4-yl)methyl]amino]phenyl]sulfonyl]-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)- | | CAS番号: | 1257044-40-8 | | 分子式: | C45H50ClN7O7S | | 分子量: | 868.44 | | EINECS: | 820-130-9 | | カテゴリ情報: | Inhibitors;Apis;Inhibitor;API;1257044-40-8 | | Mol File: | 1257044-40-8.mol | 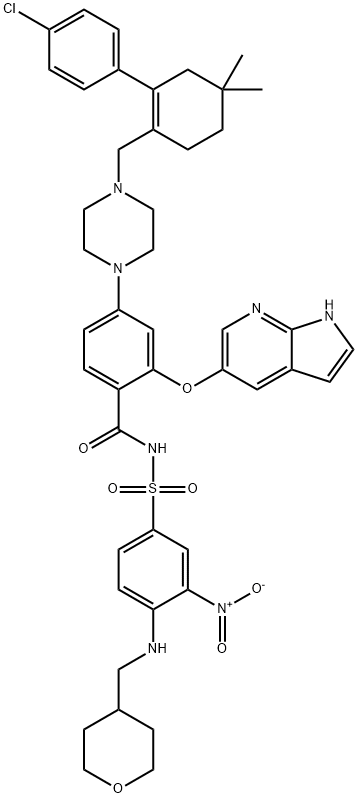 |
| 融点 | >150°C (dec.) | | 比重(密度) | 1.340±0.06 g/cm3(Predicted) | | 貯蔵温度 | -20°C Freezer | | 溶解性 | DMSO (Slightly) | | 外見 | Yellow solid. | | 酸解離定数(Pka) | 4.09±0.10(Predicted) | | 色 | Light Yellow to Yellow |
| | ベネトクラクス Usage And Synthesis |
| 効能 | 抗悪性腫瘍薬, BCL-2阻害薬 | | 説明 | Venetoclax, codeveloped by
AbbVie (previously Abbott Laboratories) and Genentech/
Roche, was approved in the US for treatment of patients with
chronic lymphocytic leukemia (CLL). To meet qualifications
for venetoclax treatment, patients must have received prior
therapy and possess the 17p deletion genetic mutation, as
determined by USFDA testing. Venetoclax functions as a
selective inhibitor of B cell lymphoma subtype 2 (BCL-2),
which is often overexpressed on malignant cells and thus leads
to impairment of the apoptotic pathway. Along these lines,
the orally dosed small molecule drug restores the ability of
malignant cells to undergo apoptosis as its mechanism of
action.90 Although other BCL-2 inhibitors are known, development
of similar agents such as navitoclox have been pursued
and halted due to undesired inhibition of BCL-XL, leading to
significant thrombocytopenia and demonstrating the need for
more selective inhibitors. Venetoclax is also currently being
considered for approval in Europe and Canada for similar
indications and is in various stages of development for the
treatment of non-Hodgkin lymphomas (NHL), acute myeloid
leukemia (AML), multiple myeloma (MM), and several other
disorders, either as a combination therapy or a stand-alone
treatment. | | 使用 | ABT 199 (>99%) is a potent and selective BCL-2 inhibitor that achieves potent antitumour activity while sparing platelets. It’s practical application is to treat chronic lymphocytic leukaemic cells and estrogen receptor-positive breast cancer. | | 定義 | ChEBI: A member of the class of pyrrolopyridines that is a potent inhibitor of the antiapoptotic protein B-cell lymphoma 2. It is used for treamtment of chronic lymphocytic leukemia with 17p deletion. | | 臨床応用 | Selective inhibitor of B-cell lymphoma protein:
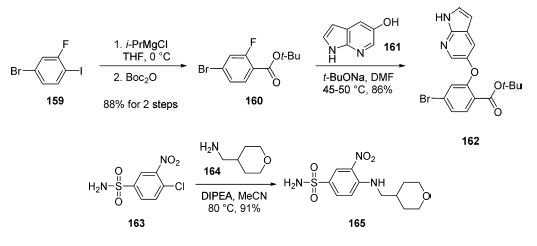
Treatment of chronic lymphocytic leukaemia | | 合成 | The manufacturing route to venetoclax takes place by
coupling of three key structural subunits: azaindole 162,
sulfonamide 165, and piperazine 172. The first of these
subunits was generated in two steps from commercially
available 4-bromo-2-fluoro-1-iodo-benzene (159).
Grignard formation of iodide 159 (i-PrMgCl) followed by
quenching with Boc2O provided the desired tert-butyl ester 160
without the need for chromatographic purification. Aromatic
substitution of crude 160 with azaindole 161 provided access to
162 in 86% yield after recrystallization from EtOAc/heptane. Sulfonamide 165 was
formed in 91% yield and 99.9% purity via aromatic substitution
of commercially available 163 with amine 164 at 80 ??C
(DIPEA, MeCN).
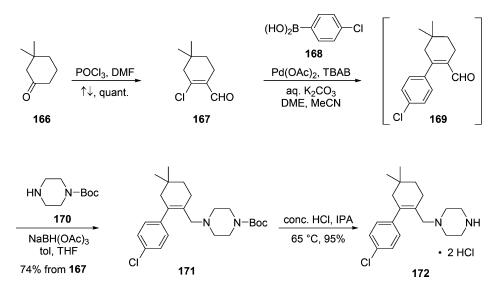
Synthesis of the third venetoclax subunit, piperazine amine
hydrochloride salt 172, began with commercial cyclohexanone
166. Vilsmeier-Haack formylation of the
sterically more accessible enol tautomer of 166 delivered vinyl
chloride 167 in quantitative yield. Coupling of this chloride
with commercial aryl boronate 168 gave rise to transient enal
169 in 87% assay yield, which was not isolated. Crude 169 was
then carried into a reductive amination reaction with
commercial N-Boc piperazine (170). Precipitation and
recrystallization from acetonitrile ultimately furnished piperazinyl
alkene 171 in 74% yield from 167. Finally, subunit 172
was obtained via Boc removal with concentrated HCl in IPA at
65 ??C and subsequent filtration, conditions that provided a 95%
yield of high purity intermediate 172 (>99.5%).
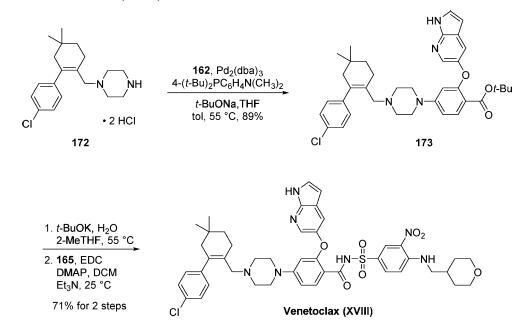
The final approach to venetoclax involved a palladiumcatalyzed
coupling of amine 172 with aryl bromide 162, ester
hydrolysis, and coupling of the resulting carboxylic acid with
sulfonamide 165. In practice, Buchwald-Hartwig amination of 162 with 172 proceeded smoothly and
relied upon workup with cysteine to enable cleansing of
residual palladium from the reaction mixture. This reaction
gave rise to advanced intermediate 173 in 89% yield after
crystallization from cyclohexane. Treatment of 173 with t-
BuOK/H2O/2-MeTHF at 55 ??C provided the corresponding
free acid, which was immediately activated with EDC/DMAP/
Et3N to promote coupling with sulfonamide 165 at room
temperature. The final drug target could be accessed by
crystallization from EtOAc and washing with 1:1 DCM/EtOAc,
yielding venetoclax (XVIII) in free base form in 71% over
the two final steps. This synthetic route was capable of
fashioning the drug target in 52% overall yield based on the
longest linear sequence (7 steps).
| | 薬物相互作用 | Potentially hazardous interactions with other drugs
Antibacterials: concentration possibly increased by
ciprofloxacin, clarithromycin and erythromycin -
reduce venetoclax dose; avoid with rifampicin.
Anticoagulants: avoid with dabigatran; concentration
of warfarin increased.
Antidepressants: avoid with St John’s wort.
Antiepileptics: concentration possibly reduced by
carbamazepine, fosphenytoin and phenytoin - avoid.
Antifungals: concentration possibly increased by
fluconazole, itraconazole, ketoconazole, posaconazole
and voriconazole - reduce venetoclax dose.
Antipsychotics: increased risk of agranulocytosis
with clozapine - avoid.
Antivirals: concentration possibly reduced by
efavirenz and etravirine - avoid; concentration
possibly increased by ritonavir - reduce venetoclax
dose.
Bosentan: concentration of venetoclax possibly
reduced by bosentan - avoid.
Calcium channel blockers: concentration possibly
increased by diltiazem and verapamil - reduce
venetoclax dose.
Cardiac glycosides: avoid with digoxin.
Cytotoxics: avoid with everolimus.
Grapefruit juice: avoid concomitant use.
Modafinil: concentration of venetoclax possibly
reduced - avoid.
Sirolimus: avoid concomitant use.
Vaccines: avoid with live vaccines. | | 代謝 | In vitro studies show that venetoclax is mainly
metabolised by cytochrome P450 CYP3A4. M27 was
identified as a major metabolite in plasma with an
inhibitory activity against BCL-2 that is at least 58-fold
lower than venetoclax in vitro.
Excretion is mainly by the faecal route (>99.9
%; 20.8
%
unchanged). | | 貯蔵 | Store at -20°C |
|