|
ChemicalBook Optimization Suppliers |
|
| | (S)-tert-Butyl 3-methyl-4-((2-nitrophenyl)sulfonyl)-1,4-diazepane-1-carboxylate 製品概要 |
| 化学名: | | | 英語化学名: | (S)-tert-Butyl 3-methyl-4-((2-nitrophenyl)sulfonyl)-1,4-diazepane-1-carboxylate | | 别名: | (S)-tert-Butyl 3-methyl-4-((2-nitrophenyl)sulfonyl)-1,4-diazepane-1-carboxylate;1H-1,4-Diazepine-1-carboxylic acid, hexahydro-3-methyl-4-[(2-nitrophenyl)sulfonyl]-, 1,1-dimethylethyl ester, (3S)-;tert-butyl (S)-3-methyl-4-[(2-nitrophenyl)sulfonyl]-1,4-diazepane-1-carboxylate;(S)-tert-Butyl 3-methyl-4-((2-nitrophenyl)sulfonyl)-1,4-diazepane-1-carboxylat | | CAS番号: | 949109-36-8 | | 分子式: | C17H25N3O6S | | 分子量: | 399.46 | | EINECS: | | | カテゴリ情報: | | | Mol File: | 949109-36-8.mol | 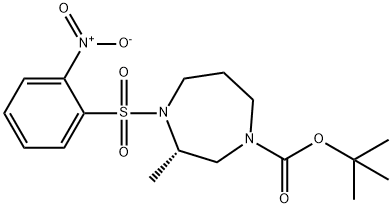 |
| | (S)-tert-Butyl 3-methyl-4-((2-nitrophenyl)sulfonyl)-1,4-diazepane-1-carboxylate 物理性質 |
| | (S)-tert-Butyl 3-methyl-4-((2-nitrophenyl)sulfonyl)-1,4-diazepane-1-carboxylate Usage And Synthesis |
| 合成 | (iv) Preparation of tert-butyl (S)-4-(2-nitrophenylsulfonyl)-3-methyl-1,4-diazepane-1-carboxylate: to a solution of (S)-3-hydroxypropyl-2-butyl ester (7.00 kg, 16.8 mol) and triphenylphosphorane (4.90 kg, 18.7 mol) in tetrahydrofuran (60 L) at 5 °C under nitrogen protection, slowly dropwise addition of Diisopropyl azodicarboxylate (4.60 kg, 22.7 mol) over 2 hours. The reaction mixture was stirred at room temperature for 7 hours. The progress of the reaction was monitored by thin layer chromatography (TLC) and after confirming complete consumption of the raw materials, the reaction mixture was concentrated under reduced pressure. A solvent mixture of petroleum ether and methyl tert-butyl ether (12.5:1, v/v) was added to the concentrated residue and stirred vigorously. The precipitate was removed by filtration and the filtrate was concentrated under reduced pressure. Petroleum ether was again added to the residue, and the precipitate was collected by filtration with vigorous stirring and dried to give a yellow solid target product (5.00 kg, 74.5% yield). The product was characterized by 1H-NMR (DMSO-d6, 80 °C): δ 0.89 (3H, d, J = 6.8 Hz), 1.40 (9H, s), 1.65-1.72 (2H, m), 3.05-3.14 (2H, m), 3.25 (1H, ddd, J = 15.6, 7.0, 7.0 Hz), 3.63 (2H, dd, J = 15.6, 5.4 Hz), 3.73 (1H, ddd, J = 15.6, 4.0, 4.0 Hz), 4.22-4.30 (1H, m), 7.79-7.88 (3H, m), 7.98 (1H, dd, J = 7.6, 2.0 Hz). Melting point: 113-114°C. | | 参考文献 | [1] Patent: US2013/144054, 2013, A1. Location in patent: Paragraph 0081; 0082; 0083 |
|