|
ChemicalBook Optimization Suppliers |
|
| | ビス(トリフルオロメタンスルホニル)イミドリチウム 製品概要 |
| | ビス(トリフルオロメタンスルホニル)イミドリチウム 物理性質 |
| | ビス(トリフルオロメタンスルホニル)イミドリチウム Usage And Synthesis |
| 外観 | 白色~わずかにうすい黄色、結晶性粉末~粉末 | | 溶解性 | 水、エタノール及びアセトンに溶ける。 | | 用途 | リチューム電池用ポリマーマトリックス電解質の調整原料 | | 説明 | Lithium bis(trifluoromethylsulphonyl)imide (LiTFSI) is normally used as a p-dopant to enhance the conductivity and hole mobility of the Spiro-OMeTAD for perovskite solar cells. It is believed that The function of LiTFSI in PSCs is similar to that in solid-state dye-sensitised solar cells. | | 使用上の注意 | アルゴン封入 | | 化学的特性 | White hygroscopic powder | | 使用 | Lithium bis(trifluoromethylsulfonyl)imide is used in the preparation of chiral imidazolium salt through an anion metathesis of the corresponding triflate organic electrolyte-based lithium batteries. It finds application in the preparation of rare-earth Lewis acid catalysts. It is also useful in primary and secondary lithium cells using organic liquid electrolytes and polymer batteries. | | 一般的な説明 | Bis(trifluoromethane)sulfonimide lithium salt is a synthetic reagent. | | 燃焼性と爆発性 | Non flammable | | 合成 |
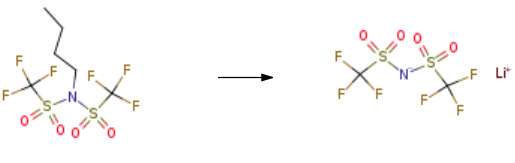
5.91 g of anhydrous lithium fluoride and N-butyl-bistrifluoromethylsulfonimide 51.26 g. Add to 250 g of isopropyl acetate and reflux for 10 hours. It was filtered after being cooled to room temperature, and the filtrate was concentrated to dryness under reduced pressure.200 g of dichloromethane was added dropwise, and the mixture was stirred at 20 ° C for 2 hours and then filtered. Wash with dichloromethane. The filter cake was dried at 80 ° C to obtain 39.57 g of Lithium bis(trifluoromethanesulphonyl)imide. The yield is 90.7%.
| | Properties and Applications | Lithium bis(trifluoromethanesulphonyl)imide (LiTFSI) is a hydrophilic organic salt with many uses in electric and electronic systems. Its bis(trifluoromethane)sulfonimide anion, often called bistriflimide, is helpful in coordinating weakly with cations. LiTFSI's other important property is its extremely high solubility in water: 21 molal or ≈6 kg/L of solution. LiTFSI is safer than the formerly used salt, lithium hexafluorophosphate (LiPF6). To improve the cells' ability to transport electrical charges, researchers are doped with a combination of LiTFSI and a semiconductor called Spiro-OMeTAD1; however, this process is extremely slow. Taylor and his fellow researchers solved the problem by bubbling carbon dioxide into a solution of spiro-OMeTAD and LiTFSI while irradiating the mixture with ultraviolet light. They then cast a film from the solution onto the perovskite light absorber. The process can be completed in ≈1 minute, compared with the older, hours-long doping procedure. |
|