|
ChemicalBook Optimization Suppliers |
| 名前: |
LGM Pharma |
| 電話番号: |
1-(800)-881-8210 |
| 電子メール: |
inquiries@lgmpharma.com |
|
| 化学名: | エソメプラゾール | | 英語化学名: | Esomeprazole | | 别名: | EsoMeprazole EC Pellets 22%;EsoMaprozole;1H-BenziMidazole,6-Methoxy-2-[(S)-[(4-Methoxy-3,5-diMethyl-2-pyridinyl)Methyl]sulfinyl]-;EsoMeprazole EC Pellets 8.5%;6-Methoxy-2-{(S)-[(4-Methoxy-3,5-diMethyl-2-pyridinyl)Methyl]sulfinyl}-1H-benziMidazole;Esomeprazole(Na、Mg);(5-methoxy-1H-benzimidazol-2-yl)[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfoniumolate;(S)-Esomeprazole | | CAS番号: | 119141-88-7 | | 分子式: | C17H19N3O3S | | 分子量: | 345.42 | | EINECS: | 643-098-6 | | カテゴリ情報: | API;119141-88-7 | | Mol File: | 119141-88-7.mol | 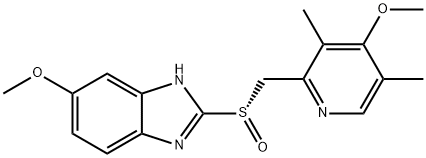 |
| 比旋光度 | D20 -155° (c = 0.5 in chloroform) | | 沸点 | 600.0±60.0 °C(Predicted) | | 比重(密度) | 1.37±0.1 g/cm3(Predicted) | | 貯蔵温度 | Sealed in dry,Room Temperature | | 溶解性 | DMF: 30 mg/ml,DMSO: 20 mg/ml,Ethanol: 10 mg/ml,PBS (pH 7.2): 10 mg/ml | | 外見 | A solid | | 酸解離定数(Pka) | 8.50±0.10(Predicted) | | CAS データベース | 119141-88-7(CAS DataBase Reference) |
| | エソメプラゾール Usage And Synthesis |
| 効能 | 胃酸分泌抑制薬, 消化性潰瘍薬, プロトンポンプ阻害薬 | | 説明 | Esomeprazole, formulated as a magnesium salt, reached the market as a treatment for
acid-related diseases such as gastro-esophageal reflux (GERD) disease including peptic
ulcer disease and reflux esophagitis. Esomeprazole (formerly perprazole) is the active (S)-
enantiomer of omeprazole (1988) and the first proton pump inhibitor developed as an
optical isomer. It can be obtained by several routes such as asymmetric oxidation of the
pro-chiral pyridylmethyl benzimidazole sulfide, separation from the racemic sulfoxide by
chiral chromatography or separation of a diastereomeric mixture obtained from the racemic
compound and a chiral acid, followed by hydrolysis. Biochemical studies have shown that
esomeprazole irreversibly inhibits the gastric H+/K+-adenosine triphosphatase (ATPase),
an enzyme system involved at the secretory surface of the stomach’s parietal cells
responsible for the secretion of gastric acid. Compared with racemic omeprazole in healthy
subjects, esomeprazole has higher bioavailability, is absorbed more rapidly and exhibits a
more uniform and predictable dose-response with higher plasma levels, leading to less
inter-individual variability between slow and rapid metabolizers. In extensive clinical trials
in patients suffering from GERD symptoms, esomeprazole provided superior acid control
and significantly reduced the healing time compared to omeprazole. | | Originator | AstraZeneca (UK) | | 使用 | (-)-Omeprazole can be used to treat migraine. | | 使用 | Esomeprazole is used to treat certain stomach and esophagus problems (such as acid reflux, ulcers). It works by decreasing the amount of acid your stomach makes. It relieves symptoms such as heartburn, difficulty swallowing, and persistent cough. | | 定義 | ChEBI: A 5-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-1H-benzimidazole that has S configuration at the sulfur atom. An inhibitor of gastric acid secretion, it is used (generally as its sodium or magnesium
alt) for the treatment of gastro-oesophageal reflux disease, dyspepsia, peptic ulcer disease, and Zollinger-Ellison syndrome. | | brand name | Nexium | | 薬物相互作用 | Potentially hazardous interactions with other drugsAnticoagulants: effect of coumarins possibly
enhanced.Antiepileptics: effects of fosphenytoin and phenytoin
enhanced.Antifungals: absorption of itraconazole and
ketoconazole reduced; avoid with posaconazole;
concentration possibly increased by voriconazole.Antivirals: concentration of atazanavir and rilpivirine
reduced - avoid concomitant use; concentration of
raltegravir and saquinavir possibly increased - avoid;
concentration of esomeprazole reduced by tipranavir.Clopidogrel: reduced antiplatelet effect.Cytotoxics: possibly reduced excretion of
methotrexate; avoid with dasatinib, erlotinib and
vandetanib; possibly reduced lapatinib absorption;
possibly reduced absorption of pazopanib.Ulipristal: reduced contraceptive effect, avoid with
high dose ulipristal | | 代謝 | Esomeprazole is completely metabolised by the cytochrome
P450 system (CYP). The major part of the metabolism of
esomeprazole is dependent on the polymorphic CYP2C19,
responsible for the formation of the hydroxy- and
desmethyl metabolites of esomeprazole. The remaining
part is dependent on another specific isoform, CYP3A4,
responsible for the formation of esomeprazole sulphone,
the main metabolite in plasma. The major metabolites of
esomeprazole have no effect on gastric acid secretion.Almost 80% of an oral dose of esomeprazole is excreted
as metabolites in the urine, the remainder in the faeces.
Less than 1% of the parent drug is found in urine. |
|