|
ChemicalBook Optimization Suppliers |
| 名前: |
Energy Chemical |
| 電話番号: |
021-021-58432009 400-005-6266 |
| 電子メール: |
sales8178@energy-chemical.com |
|
| 化学名: | ナフテン酸 | | 英語化学名: | NAPHTHENIC ACID | | 别名: | NAPHTENIC ACID;NAPHTHENIC ACID;carboxylicacids,naphthenic;naphid;NAPHTHENICACIDANDITSSODIUM,CALCIUMANDMANGANESESALTS;sunaptic acid c;NAPHTHENIC ACID, ACID VALUE 220-230;cyclohexanecarbaroxylic acid | | CAS番号: | 1338-24-5 | | 分子式: | C7H10O2 | | 分子量: | 126.154 | | EINECS: | 215-662-8 | | カテゴリ情報: | | | Mol File: | 1338-24-5.mol | 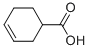 |
| 沸点 | 160-198 °C (6 mmHg) | | 比重(密度) | 0.92 g/mL at 20 °C (lit.) | | 蒸気圧 | 31.4Pa at 25℃ | | 屈折率 | n20/D 1.45 | | 闪点 | 149 °C | | 溶解性 | Practically insoluble in water | | 酸解離定数(Pka) | 5[at 20 ℃] | | 外見 | Liquid | | 色 | Clear colorless to yellow-brown | | 水溶解度 | 88.1mg/L at 20℃ | | 凝固点 | -30~-36℃ | | InChIKey | VUSWCWPCANWBFG-UHFFFAOYSA-N | | LogP | 7.65 | | EPAの化学物質情報 | Naphthenic acids (1338-24-5) |
| | ナフテン酸 Usage And Synthesis |
| 外観 | わずかにうすい黄色~黄褐色, 液体 | | 溶解性 | 水に不溶。有機溶剤に可溶。エタノールに極めて溶けやすく、水にほとんど溶けない。 | | 解説 | ナフテン酸,金属塩として,乳化剤(ナトリウム,カリウム塩),極圧添加剤(鉛,アルミニウム塩),ペイント乾燥剤(鉛,マンガン,亜鉛塩),殺菌剤(銅塩),油溶性酸化触媒(コバルト,マンガン塩)など,多くの用途に使用される. | | 製法 | ナフテン酸,ナフテン環をもつ環状飽和脂肪酸.石油に含まれる有機酸素化合物の主要部を占め,石油のアルカリ処理液の中和で得られる石油酸から精製される.各種の構造のものがあるが,次の一般式に属するタイプがもっとも多い. | | 主な用途/役割 | ホットメルト接着剤の可塑剤として使用される。 | | 化学的特性 | CLEAR YELLOWISH TO BROWN VISCOUS LIQUID | | 化学的特性 | Naphthenic acid is a gold to black, odorless
liquid. | | 使用 | Naphthenic acid is commonly used in the synthesis of useful metal naphthenates such as copper naphthenate, a wood preservative; titanium naphthenate, a precursor for the preparation of titanium oxide thin films and a rare earth naphthenate, a lubricant oil additive. It can also be in the synthesis of biodegradable naphthenic acid ionic liquids. | | 使用 | The term naphthenic acid, as commonly used in the petroleum industry, refers collectively to all of the carboxylic acids present in crude oil. | | 一般的な説明 | NAPHTHENIC ACID is a dark colored liquid with an offensive odor. NAPHTHENIC ACID is insoluble in water. NAPHTHENIC ACID will burn though NAPHTHENIC ACID may take some effort to ignite. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Since NAPHTHENIC ACID is a liquid NAPHTHENIC ACID can easily penetrate the soil and contaminate groundwater and nearby streams. NAPHTHENIC ACID is used to make paint dryers, detergents, and solvents. | | 空気と水の反応 | Insoluble in water. | | 反応プロフィール | NAPHTHENIC ACID is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in NAPHTHENIC ACID to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. | | 健康ハザード | Principal effect is that of mild primary irritation when encountered in high concentrations. Inhalation of vapor causes coughing. Liquid is moderately irritating to eyes and slightly to moderately irritating to skin; excessive exposure could result in dermatitis. | | 燃焼性と爆発性 | Not classified | | 安全性プロファイル | Moderately toxic by
ingestion and intraperitoneal routes. When
heated to decomposition it emits acrid
smoke and irritating fumes. | | 職業ばく露 | Used to make metallic naphthenates
for paint dryers and cellulose preservatives. It is also used
as a solvent; detergent, rubber reclaiming agent. Used in
catalysts, cutting oils; drilling compounds; rust inhibitors;
surfactants, emulsions, grease, and wood preservatives. | | 輸送方法 | UN3082 Environmentally hazardous substances,
liquid, n.o.s., Hazard Class: 9; Labels: 9-Miscellaneous
hazardous material, Technical Name Required. | | 不和合性 | Compounds of the carboxyl group react
with all bases, both inorganic and organic (i.e., amines)
releasing substantial heat, water, and a salt that may be
harmful. Incompatible with arsenic compounds (releases
hydrogen cyanide gas), diazo compounds, dithiocarbamates,
isocyanates, mercaptans, nitrides, and sulfides
(releasing heat, toxic, and possibly flammable gases), thiosulfates
and dithionites (releasing hydrogen sulfate and
oxides of sulfur). Incompatible with sulfuric acid, caustics,
ammonia, aliphatic amines; alkanolamines, isocyanates,
alkylene oxides; epichlorohydrin, strong oxidizers.
Corrosive to metals. |
|