| Company Name: |
Energy Chemical
|
| Tel: |
021-58432009 400-005-6266 |
| Email: |
marketing@energy-chemical.com |
| Products Intro: |
Product Name:Anirolac
CAS:66635-85-6
Purity:NULL Package:25mg;50mg Remarks:NULL
|
| Company Name: |
CLEARSYNTH LABS LTD.
|
| Tel: |
+91-22-45045900 |
| Email: |
sales@clearsynth.com |
| Products Intro: |
Product Name:Anirolac
CAS:66635-85-6
|
|
| | Anirolac Basic information |
| Product Name: | Anirolac | | Synonyms: | Anirolac;2,3-Dihydro-5-(4-methoxybenzoyl)-1H-pyrrolizine-1-carboxylic acid;1H-Pyrrolizine-1-carboxylic acid, 2,3-dihydro-5-(4-methoxybenzoyl)-;RS 37326 | | CAS: | 66635-85-6 | | MF: | C16H15NO4 | | MW: | 285.29 | | EINECS: | | | Product Categories: | | | Mol File: | 66635-85-6.mol | 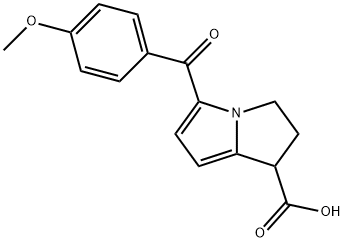 |
| | Anirolac Chemical Properties |
| Boiling point | 532.6±50.0 °C(Predicted) | | density | 1.33±0.1 g/cm3(Predicted) | | pka | 4.27±0.20(Predicted) |
| | Anirolac Usage And Synthesis |
| Originator | Anirolac,Syntex Inc. | | Uses | Antiinflammatory; analgesic. | | Uses | Anirolac is a nonsteroidal anti-inflammatory drug with analgesic properties. Study suggests that pain relief due to Anirolac is equivalent to that of Naproxen (N377520). | | Manufacturing Process | A solution of 1.1 equivalent of N,N-dimethyl-p-methoxybenzamide and 1 equivalent of phosphorous oxychloride in 2 ml of 1,2-dichloroethane is
refluxed for 30 minutes. To this solution is added a solution of 1 equivalent of
isopropyl 1,2-dihydro-3H-pyrrolo[1,2-a]pyrrole-1-carboxylate in 2 ml of 1,2-
dichloroethane. The reaction mixture is refluxed under an argon atmosphere
for 8 hours, treated with equivalent of sodium acetate and refluxed for a
further 5 hours. The resultant mixture is then evaporated to dryness and the
residue is chromatographed on 12 g of silica gel, eluting with hexane: ethyl
acetate (3:1), monitoring the course of the reaction by TLC. Isopropyl 5-pmethoxybenzoyl-
1,2-dihydro-3H-pyrrolo[1,2-a]pyrrole-1-carboxylate was
obtained as an oil. UV, IR, NMR spectrum confirmed the structure of obtained
compound.
A solution of 1 equivalent of isopropyl 5-p-methoxybenzoyl-1,2-dihydro-3Hpyrrolo[
1,2-a]pyrrole-1-carboxylate in 10 ml of methanol is treated with a
solution of 1 equivalent of potassium carbonate in 5 ml of water. The reaction
mixture is refluxed under nitrogen atmosphere for 30 minutes, cooled, and
evaporated to dryness. The residue is taken up in 10 ml of 10% aqueous
hydrochloric acid and 50 ml of water and the resultant mixture extracted with
ethyl acetate (2x50 ml). The combined extracts are dried over magnesium
sulfate and evaporated to dryness under reduced pressure. Crystallization of
the residue from ethyl acetate-hexane affords 5-p-methoxybenzoyl-1,2-
dihydro-3H-pyrrolo[1, 2-a]pyrrole-1-carboxylic acid (anirolac); MP: 187°-
187.5°C. | | Therapeutic Function | Antiinflammatory, Analgesic |
| | Anirolac Preparation Products And Raw materials |
|