|
ChemicalBook Optimization Suppliers |
|
| 化学名: | バリノマイシン | | 英語化学名: | VALINOMYCIN | | 别名: | 1,7,13,19,25,31-hexaoxa-4,10,16,22,28,34-hexaazacyclohexatriacontane-2,5,8,11,;14,17,20,23,26,29,32,35-dodecone,12,24,36-trimetyl-3,6,9,15,18,21,27,30,33-non;akis(1-methylethyl)-[qr];aleryl-d-valyl-l-lactoyl-l-valyl-d-alpha-hydroxyisovaleryl-d-valyl-l-lactoyl-l;antibioticn-329b;cyclic(d-alpha-hydroxyisovaleryl-d-valyl-l-lactoyl-l-valyl-d-alpha-hydroxyisov;nsc122023;nsc122023[qr] | | CAS番号: | 2001-95-8 | | 分子式: | C54H90N6O18 | | 分子量: | 1111.32 | | EINECS: | 217-896-6 | | カテゴリ情報: | antibiotic;Agro-Products;Inhibitors;Peptides | | Mol File: | 2001-95-8.mol | 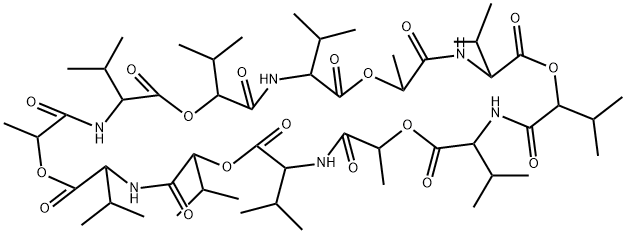 |
| 融点 | 187-190 °C | | 比旋光度 | D20 +31.0° (c = 1.6 in benzene) | | 沸点 | 821.74°C (rough estimate) | | 比重(密度) | 1.060 | | 屈折率 | 1.6400 (estimate) | | 闪点 | 87℃ | | 貯蔵温度 | 2-8°C | | 溶解性 | DMSO: ≥10 mg/mL | | 外見 | solid | | 酸解離定数(Pka) | 11.32±0.70(Predicted) | | 色 | white | | 光学活性 (optical activity) | [α]20/D +32±2°, c = 1.6% in benzene | | 水溶解度 | Soluble in DMSO at 10mg/ml. Insoluble in water | | Merck | 13,9976 | | BRN | 78657 | | 安定性: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 3 months. | | EPAの化学物質情報 | Valinomycin (2001-95-8) |
| | バリノマイシン Usage And Synthesis |
| 外観 | 白色~ほとんど白色、結晶性粉末~粉末 | | 溶解性 | クロロホルムに可溶。水に不溶。有機溶剤に可溶。クロロホルム及びメタノールに溶ける。 | | 解説 | バリノマイシン,放線菌から得られる抗生物質の一種で,[ (D-バリン)-(L-乳酸)-(L-バリン)-(D-α-ヒドロキシイソ吉草酸) ]3 という 12個の単位が環状につながっている。抗生物質としてはグラム陽性菌に有効である。高等生物に作用させると細胞膜やミトコンドリア膜のカリウム透過性を選択的に高めるので,イオン輸送などにおける研究手段としてしばしば用いられる。バリノマイシンの輪が膜に入り込んでチャンネルを形成し,そこを,大きさの合致するカリウムイオンが通り抜けるものと考えられる。 | | 用途 | 生体膜研究用、生理作用研究用。 | | 説明 | Valinomycin (2001-95-8) is a selective K+ ionophore capable of transporting potassium ions through lipid membranes.1 Valinomycin can induce apoptosis in cells by causing a rapid loss of mitochondrial membrane potential via potassium efflux.2,3 | | 化学的特性 | white crystalline powder | | 使用 | antibiotic; LD50 (rat, po) 4 mg/kg | | 使用 | Insecticide, nematocide, Patterson, Wright, US 3520973 (1970 to Am. Cyanamid). | | 使用 | K+-selective ionophoric cyclodepsipeptide; potassium ionophore which uncouples oxidative phosphorylation, induces apoptosis in murine thymocytes, inhibits NGF-induced neuronal differentiation and anta
gonizes ET-induced vasoconstriction. Used as insecticide, nematocide. | | 使用 | Valinomycin is a hydrophobic cyclodepsipeptide with potent antitumor activity. Valinomycin is a highly selective potassium ionophore and this action leads to a diverse range of profound cell membrane effects. More recently, valinomycin has found application as a biosensor to detect to detect potassium efflux. | | 使用 | Valinomycin is a hydrophobic cyclodepsipeptide with potent antitumour activity. Valinomycin is a highly selective potassium ionophore and this action leads to a diverse range of profound cell membrane effects. More recently, valinomycin has found application as a biosensor to detect to detect potassium efflux. | | 定義 | ChEBI: A twelve-membered cyclodepsipeptide composed of three repeating D-alpha-hydroxyisovaleryl-D-valyl-L-lactoyl-L-valyl units joined in sequence. An antibiotic found in severa
Streptomyces strains. | | 一般的な説明 | Shiny crystalline solid. Used as an insecticide and nematocide. Not registered as a pesticide in the U.S. | | 反応プロフィール | VALINOMYCIN is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). | | 健康ハザード | VALINOMYCIN is highly toxic orally. | | 火災危険 | When heated to decomposition, VALINOMYCIN emits toxic fumes of nitrogen oxides. | | 生物活性 | Selective K + ionophore (K 0.5 values are 48, 73, 75, 93 and 246 mM for K + , Rb + , Cs + , Na + and Li + respectively) that transports K + across biological and artificial lipid membranes. Inhibits Ca 2+ -ATPase activity and induces apoptosis through mitochondrial membrane depolarization, caspase-3 activation and phosphatidylserine translocation in vitro . | | Biochem/physiol Actions | Valinomycin affects the ion transport behavior of mitochondrial systems. | | in vitro | valinomycin caused substantial cho cells death within 12 h of treatment. several apoptotic events were identified in valinomycin-treated cho cells, including caspase-3 activation, phosphatidylserine (ps) membrane translocation, and mitochondrial membrane depolarization during the first few hours of treatment. k+ efflux was reduced by elevating extracellular k+ concentrations [2]. | | in vivo | valinomycin is irritant in the case of eye and skin contact. inhalation of valinomycin can cause breathing disturbances and even loss of conscious. lethal doses (ld50) for mouse and rabbit is 2.5 mg/kg and 5 mg/kg respectively. valinomycin also shows to provoke lots of chronic effects, including damage of the central and peripheral nervous system, eyes, lens and cornea [1]. | | 貯蔵 | Store at -20°C | | 純化方法 | Recrystallise valinomycin from dibutyl ether or Et2O. It is dimorphic: modification A crystallises from n-octane, and modification B crystallises from EtOH/H2O. It is soluble in pet ether, CHCl3, AcOH, BuOAc and Me2CO. [Smith et al. J Am Chem Soc 97 7242 1975, UV, IR and NMR see Brocknmann & Schmidt-Kastner Chem Ber 88 57 1955, Beilstein 27 I 9728. 17 IV 9728.] | | 参考文献 | 1) Pressman, (1976) Biological applications of ionophores; Annu. Rev. Biochem., 45 501
2) Furlong et al., (1998) Induction of apoptosis by valinomycin: mitochondrial permeability transition causes intracellular acidification; Cell Death Diff., 5 214
3) Inai et al. (1997) Valinomycin induces apoptosis of ascites hepatoma cells (AH-130) in relation to mitochondrial membrane potential; Cell Struc. Funct., 22 555 |
|