|
ChemicalBook Optimization Suppliers |
| 名前: |
Alfa Aesar |
| 電話番号: |
400-6106006 |
| 電子メール: |
saleschina@alfa-asia.com |
| 名前: |
Energy Chemical |
| 電話番号: |
021-021-58432009 400-005-6266 |
| 電子メール: |
sales8178@energy-chemical.com |
|
| 化学名: | フェノキシナトリウム | | 英語化学名: | Sodium benzenolate | | 别名: | Phenol,sodiumsalt;phenol,sodiumsalt(solid);phenolatesodium;Phenolsodium;phenolsodiumsalt;sodiumcarbolate;sodiumphenolate,solid;SODIUM PHENYL | | CAS番号: | 139-02-6 | | 分子式: | C6H5NaO | | 分子量: | 116.09 | | EINECS: | 205-347-3 | | カテゴリ情報: | | | Mol File: | 139-02-6.mol | 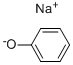 |
| 融点 | >300℃ | | 比重(密度) | 0,898 g/cm3 | | RTECS 番号 | SM8780000 | | 闪点 | 28°C | | 貯蔵温度 | below 5° C | | 溶解性 | DMSO (Slightly, Sonicated), Methanol (Slightly) | | 外見 | Solid | | 色 | Pale Yellow to Pale Brown | | 比重 | 0.898 | | 水溶解度 | Very soluble in water, soluble in alcoholSoluble in water, acetone and alcohol. | | Sensitive | Hygroscopic | | 安定性: | Hygroscopic | | CAS データベース | 139-02-6(CAS DataBase Reference) | | EPAの化学物質情報 | Sodium phenate (139-02-6) |
| Provider | Language |
|
ALFA
| English |
| | フェノキシナトリウム Usage And Synthesis |
| 効能 | 殺菌消毒薬 | | 化学的特性 | Sodium phenate, or sodium phenoxide, NaOC6H5, is a white crystalline substance that easily dissolves in water and alcohol. It decomposes when exposed to carbon dioxide in the air. It is produced by reacting sodium hydroxide solution (not carbonate) with phenol and subsequently evaporating the mixture. Sodium phenate is commonly used in the production of sodium salicylate. | | 使用 | As a general disinfectant, either in solution or mixed with slaked lime, etc., for toilets, stables, cesspools, floors, drains, etc.; for the manufacture of colorless or light-colored artificial resins, many medical and industrial organic Compounds and dyes; as a reagent in chemical analysis. Pharmaceutic aid (preservative). | | 使用 | Sodium phenoxide is used as a precursor to aryl ethers and involved in the preparation of phenyl ethers and metal phenolates. It serves as an antiseptic and used in organic synthesis. | | 定義 | ChEBI: Sodium phenolate is a phenolate. It has a role as a disinfectant. | | 製造方法 | Sodium phenoxide was freshly prepared by adding sodium metal to phenol (0.45 g, 4.8 mmol) dissolved in diethyl ether (15 cm3), under an atmosphere of dry nitrogen. This was refluxed with (11) (1.0 g, 2.8 mmol) for 23 h. Following evaporation of the ether, water (20 cm3) was added and the mixture extracted into DCM. The DCM solution was dried (MgS04) and evaporated to give a solid (1.2 g). Fractional sublimation and recrystallisation from acetonitrile gave recovery of 2,4,6-tribromo-3,5-difluoropyridine (0.8 g, 80%) and 3-phenoxy-5-fluoro-2,4,6-tribromopyridine (0.2 g, 20%), m.p. 100±1 OC (Found: C, 31.3; H, 1.1; N, 2.9. C11H5Br3FNO requires C, 31.0; H, 1.2; N, 3.3%); IR spectrum no. 15; mass spectrum no. 15; nmr spectrum no. 19. | | 一般的な説明 | A white to reddish colored solid in the form of crystalline rods. Used as an antiseptic and in organic synthesis. | | 空気と水の反応 | Decomposes in air. Soluble in water. In both cases, a corrosive alkaline solution forms that is a strong irritant to skin and eyes. | | 反応プロフィール | Salts, basic, such as SODIUM PHENOLATE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. | | 危険性 | Strong irritant to skin and tissue. | | 安全性プロファイル | Poison by subcutaneous route. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of Na2O. See also PHENOL and SODIUM HYDROXIDE. | | 概要 | フェノール類は弱酸なので、水酸化ナトリウムのような塩基の水溶液と反応して塩をつくり、水に溶ける。 | | 純化方法 | The ground powder is washed with Et2O, then heated at 60o/1mm for 12 to 24hours to remove any free phenol and solvent. [Kornblum & Lurie J Am Chem Soc 81 2710 1959, Beilstein 6 I 718.] |
|