|
ChemicalBook Optimization Suppliers |
|
| 融点 | 158°C | | 沸点 | 430.6±45.0 °C(Predicted) | | 比重(密度) | 1.471±0.06 g/cm3(Predicted) | | 貯蔵温度 | -20°C | | 溶解性 | DMSO: soluble20mg/mL, clear | | 酸解離定数(Pka) | 9.89±0.70(Predicted) | | 外見 | powder | | 色 | white to beige | | Merck | 14,8249 | | 安定性: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. | | InChI | InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | | InChIKey | MNDBXUUTURYVHR-UHFFFAOYSA-N | | SMILES | C(NC1C(Cl)=CN=CC=1Cl)(=O)C1=CC=C(OC(F)F)C(OCC2CC2)=C1 | | CAS データベース | 162401-32-3(CAS DataBase Reference) |
| 主な危険性 | Xi | | Rフレーズ | 36/37/38 | | Sフレーズ | 26 | | WGK Germany | 3 | | RTECS 番号 | CV3325600 | | HSコード | 2933.39.4100 |
| | ロフルミラスト Usage And Synthesis |
| 外観 | 白色~黄赤色~緑色粉末~結晶 | | 効能 | 喘息治療薬, ホスホジエステラーゼ4阻害薬 | | 説明 | Roflumilast is a selective, orally active PDE4 inhibitor thatwas approved
in Germany in July 2010 as an add-on to bronchodilator treatment for
maintenance therapy of severe chronic obstructive pulmonary disorder
(COPD) associated with chronic bronchitis in adult patients with a history
of frequent exacerbations .
Roflumilast and its primary metabolite roflumilast N-oxide are potent and
competitive inhibitors of PDE4 and are equipotent against PDE4A, B, andD
but inactive against PDE4C and the other ten members of the PDE family
(PDEs 1–3, 5–11). Despite its inhibition of PDE4D (IC50=0.80 nM, N-oxide
IC50=2.0 nM), roflumilast shows the lowest incidence of nausea (3–5%)
among the PDE4 inhibitors investigated in clinical trials.Anti-inflammatory
effects of roflumilast have been demonstrated in preclinical cellular and
animal models. Roflumilast is synthesized in four steps from
3-(cyclopropylmethoxy)-4-hydroxybenzaldehyde. The difluoromethyl
ether is introduced by alkylation of the free phenolic group with
chlorodifluoromethane and base. The aldehyde moiety is oxidized to the
benzoic acid, which is then converted to an acid chloride and coupled with
3,5-dichloro-4-aminopyridine.
Roflumilast is rapidly absorbed and metabolized to its active metabolite,
roflumilast N-oxide. Metabolism is mediated by CYP3A4
and CYP1A2. | | 説明 | Type 4 cyclic nucleotide phosphodiesterase (PDE4) isoforms selectively inactivate the second messenger cAMP by hydrolyzing the phosphodiester bond, producing AMP. Roflumilast is a potent, cell-permeable inhibitor of PDE4 (IC50 = < 1 nM for both human PDE4B and PDE4D). It is selective for PDE4, with IC50 values against other PDE forms being greater than 10 μM. Roflumilast demonstrates good bioavailability and has applications in respiratory diseases, including asthma and chronic obstructive pulmonary disease. | | 化学的特性 | Crystallin Solid | | Originator | BYK Gulden Lomberg Chemische Fabrik GmbH (Germany) | | 使用 | Selective phosphodiesterase 4(PDE4) inhibitor. Antiasthmatic; in treatment of chronic obstructive pulmonary disease | | 使用 | Roflumilast is a selective, long-acting PDE-4 inhibitor approved in 2010 for the treatment of
inflammatory conditions of the lungs such as asthma and chronic obstructive pulmonary disorder. Marketed under the trade name Daxas?, roflumilast was developed by researchers at the
University of Liverpool in partnership with Nycomed. Although the dose-limiting side effects of the
drug are mild nausea, diarrhea, and weight loss, these symptoms subsided after a few weeks of
treatment. | | 使用 | Roflumilast (Daxas) is a selective inhibitor of PDE4 with IC50 of 0.2-4.3 nM. | | 使用 | ophthalmic solution | | 定義 | ChEBI: A benzamide obtained by formal condensation of the carboxy group of 3-(cyclopropylmethoxy)-4-(difluoromethoxy)benzoic acid with the amino group of 3,5-dichloropyridin-4-amine. Used for treatment of bronchial asthma and chronic obstructive pulmonary disease | | brand name | Daxas | | Biochem/physiol Actions | Roflumilast is a highly potent, orally active, and selective phosphodiesterase 4 (PDE4) inhibitor with an IC50 of 0.8 nM. Roflumilast has anti-inflammatory properties and is used clinically to treat COPD. | | 作用機序 | Roflumilast is the more potent of the two drugs, and along with its active metabolite, roflumilast-N-oxide, it is nonselective in its
inhibitory action on PDE4B and PDE4D. The PDE4B appears to be the most closely linked to anti-inflammatory effects, whereas the
PDE4D receptor subtype is thought to be linked to nausea, possibly through a central effect. Roflumilast exhibits 80% oral
bioavailability and has an elimination half-life of 10 hours, whereas the N-oxide has an elimination half-life of 20 hours and has shown
no drug interactions. Clinical trials in patients with asthma or COPD are quite promising. | | 薬物動態学 | Roflumilast is well absorbed on oral administration
and has a half-life of 10 hours. Roflumilast is metabolized in the liver to its N-oxide derivative,
which also is a PDE4 inhibitor, and it has a plasma half-life of 20 hours. | | 臨床応用 | Roflumilast is currently undergoing clinical trials in Europe for use in the treatment of both
asthma and COPD. | | 合成 | The straightforward preparation of roflumilast begins with commercially available
methyl 3,4-dihydroxybenzoate (130). Alkylation of the more reactive 3-
hydroxyl group with (bromomethyl)cyclopropane (131) preceded a second alkylation of the remaining
p-phenol with chlorodifluoromethane in aqueous sodium hydroxide. These phase-transfer conditions
saponified the ester within 130 and after acidic quench, carboxylic acid 132 was ultimately furnished in
excellent yield (97%) over the three step protocol. Activation of 132 as the corresponding acyl halide
through use of thionyl chloride (SOCl2) and subsequent exposure to commercial aminopyridine 133
provided roflumilast (XII) in 81% yield. 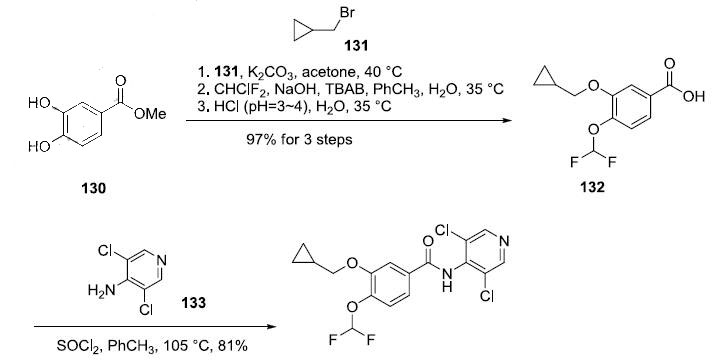
| | target | cAMP | ROS | IL Receptor | PDE | | 貯蔵 | Store at +4°C | | 参考文献 | 1) Hatzelmann?et al.?(2010),?The preclinical pharmacology of roflumilast—a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease; Pulm, Pharmacol. Ther.,?23?235
2) Rabe?et al.?(2011),?Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease; Br. J. Pharmacol,?163?53
3) Heckman?et al.?(2018),?Acute administration of roflumilast enhances sensory gating in healthy young humans in a randomized trial; Psychopharmacology (Berl.),?235?301
4) Vanmierlo?et al.?(2016),?The PDE4 inhibitor roflumilast improves memory in rodents at non-emetic doses;?Behav. Brain Res.,?303?26
5) Tikoo?et al.?(2014),?Calorie restriction mimicking effects of roflumilast prevents diabetic nephropathy; Biochem. Biophy. Res. Commun.,?450?1581
6) Mollmann?et al.?(2017),?The PDE4 inhibitor roflumilast reduced weight gain by increasing energy expenditure and leads to improved glucose metabolism; Diabetes Obes. Metab.,?19?496 |
|