|
ChemicalBook Optimization Suppliers |
|
| 化学名: | メトリホネート | | 英語化学名: | Trichlorfon | | 别名: | O,O-Dimethyl-(2,2,2-trichloor-1-hydroxy-ethyl)-fosfonaat;O,O-Dimethyl-(2,2,2-trichlor-1-hydroxy-aethyl)phosphonat;o,o-dimethyl(2,2,2-tri-chloro-1-hydroxyethyl)phosphonate;o,o-dimethyl(2,2,2-trichloro-1-hydroxyethyl)phosponate;o,o-dimethyl-(2,2,2-trichloro-1-idrossi-etil)-fosfonato;o,o-dimethyl-1-oxy-2,2,2-trichloroethylphosphonate;O,O-Dimetil-(2,2,2-tricloro-1-idrossi-etil)-fosfonato;o,odimetil2,2,2-trichloro1hidroxietilfosfonato | | CAS番号: | 52-68-6 | | 分子式: | C4H8Cl3O4P | | 分子量: | 257.44 | | EINECS: | 200-149-3 | | カテゴリ情報: | SANCTURA;Bases & Related Reagents;Carbohydrates & Derivatives;Isotope Labeled Compounds;Nucleotides;Phosphorylating and Phosphitylating Agents;INSECTICIDE | | Mol File: | 52-68-6.mol | 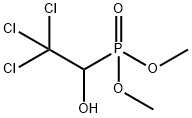 |
| 融点 | 77-81 °C | | 沸点 | 100°C | | 比重(密度) | 1.73 | | 蒸気圧 | 2.1×10-4Pa (20 °C) | | 屈折率 | 1.3439 | | 貯蔵温度 | 2-8°C | | 溶解性 | Freely soluble in water, very soluble in methylene chloride, freely soluble in acetone and in ethanol (96 per cent). | | 外見 | solid | | 酸解離定数(Pka) | 6 (est.) | | 色 | Crystals | | 水溶解度 | Slightly soluble. 1-5 g/100 mL at 21 ºC | | Merck | 13,9696 | | BRN | 1709434 | | 安定性: | Light Sensitive | | IARC | 3 (Vol. 30, Sup 7) 1987 | | NISTの化学物質情報 | Metrifonate(52-68-6) | | EPAの化学物質情報 | Trichlorfon (52-68-6) |
| | メトリホネート Usage And Synthesis |
| 外観 | 白色, 結晶~粉末 | | 溶解性 | 水に溶けやすい。 | | 用途 | 農薬 (殺虫剤) (化学工業日報社) | | 効能 | 駆虫薬 (獣医薬), アセチルコリンエステラーゼ阻害薬 | | 農薬用途 | 動物薬、殺虫剤 | | 説明 | Trichlorfon is a colorless crystalline powder.
It is soluble in water (120 g/L) and most organic solvents,
except aliphatic hydrocarbons. Log Kow = 0.43. Trichlorfon
is rapidly converted to dichlorvos by alkalis (2) and then
hydrolyzed; DT50 (22 ?C) values at pH 4, 7, and 9 are 510 d,
46 h, and <30 min, respectively. | | 化学的特性 | White or almost white, crystalline powder. | | 化学的特性 | Trichlorfon is a white to pale yellow crystalline solid. | | 使用 | Insecticide used to control ?ies and roaches. | | 使用 | anticholinergic, urinary incontenance therapy | | 使用 | One of the biologically active forms of nicotinic acid. Differs from NAD by an additional phosphate group at the 2?position of the adenosine moiety. Serves as a coenzyme of hydrogenases and dehydrogenases. Present in living cells primarily in the r | | 使用 | Trichlorfon is used to control a wide range of insects in many crops
and to control household pests, flies in animal houses and ectoparasites
in domestic animals. | | 使用 | Trichlorfon is an irreversible organophosophate acetylcholinesterase inhibitor and the prodrug of Dichlorvos (D435950). Trichlorfon have also shown potential actions to be utilized as an effective org
anophosphorus pesticide. | | 定義 | ChEBI: A phosphonic ester that is dimethyl phosphonate in which the hydrogen atom attched to the phosphorous is substituted by a 2,2,2-trichloro-1-hydroxyethyl group. | | 適応症 | Metrifonate is an organophosphorous compound that
is effective only in the treatment of S. haematobium.
The active metabolite, dichlorvos, inactivates acetylcholinesterase
and potentiates inhibitory cholinergic effects.
The schistosomes are swept away from the bladder
to the lungs and are trapped. Therapeutic doses
produce no untoward side effects except for mild
cholinergic symptoms. It is contraindicated in pregnancy,
previous insecticide exposure, or with depolarizing
neuromuscular blockers. Metrifonate is not available
in the United States. | | 抗菌性 | Useful activity is restricted to Schistosoma haematobium. It
has little activity against other schistosomes.
Although it exhibits activity against several other helminths, it
is not used for their treatment. | | 一般的な説明 | Metrifonate is an organophosphate thatwas originally developed to treat schistosomiasis under thetrade name Bilarcil. It is an irreversible cholinesteraseinhibitor with some selectivity for BuChE over AChE. Itachieves sustained cholinesterase inhibition by its nonenzymaticmetabolite dichlorvos (DDVP), a long-actingorganophosphate. Its use in mild-to-moderate Alzheimerdisease was suspended recently because of adverse effectsexperienced by several patients during the clinical evaluationof this product. Toxicity at the neuromuscular junctionis probably attributable to the inhibition by the drug of neurotoxicesterase, a common feature of organophosphates. | | 一般的な説明 | Chlorophos is a white crystalline solid. Soluble in water, benzene, chloroform, ether; insoluble in oils. Chlorophos is a wettable powder. Chlorophos can cause illness by inhalation, skin absorption and/or ingestion. Chlorophos is used as a pesticide. | | 空気と水の反応 | Chlorophos decomposes at higher temperatures in water and at pH <5.5. Chlorophos is sensitive to prolonged exposure to moisture. Chlorophos is unstable in alkaline solutions. | | 反応プロフィール | Chlorophos is incompatible with alkalis. Chlorophos is corrosive to black iron and mild steel. Chlorophos is corrosive to metals. Chlorophos is subject to hydrolysis. | | 健康ハザード | INHALATION, INGESTION, AND SKIN ABSORPTION. Inhibits cholinesterase. Headache, depressed appetite, nausea, miosis are symptoms of light exposures. Moderate effects are peritoneal paralysis, diarrhea, salivation, lacrimation, sweating, dyspnea, substernal tightness, slow pulse, tremors, muscular cramps and ataxia. Severe symptoms are: pyrexia, cyanosis, pulmonary edema, areflexia, loss of sphincter control, paralysis, coma, heart block, shock and respiratory failure. EYES: Increases permeability of blood vessels in anterior eye. Reduces corneal sensitivity with glaucoma, abnormalities in intraocular tension or decreased visual acuity. | | 火災危険 | Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form. | | 农业用途 | Insecticide, Anthelmintic: Not approved for use in EU countries
. Registered
for use in the U.S. except California. Trichlorfon has
non-agriculture uses on golf course turf, home lawns and
similar venues, and in non-food contact areas of food and
meat processing plants. Also on ornamental shrubs and
plants, and ornamental and bait fish ponds. Overseas, trichlorfon is used as cattle pour-on, which is classified as a
food-use. It is used against insects such as lepidopteran larvae (caterpillars), white grubs, mole crickets, cattle lice, sod
webworms, leaf miners, stink bugs, flies, ants, cockroaches,
earwigs, crickets, diving beetles, water scavenger beetles,
water boatman, backswimmers, water scorpions, giant water bugs and pillbugs. All food and feed uses in the U.S.
were voluntarily canceled November 21, 1995. It was used
on Brussels sprouts, barley, beets, blueberries, beans (dryand snap), corn, field corn, popcorn, sweet corn, cotton,
cow peas, lima beans, tomatoes, cabbage, carrots (including
tops), cauliflower, collards, cowpeas, southern peas, blackeyed peas, crowder peas, pumpkins, collards, lettuce and
alfalfa, cotton, peanuts, peppers, pumpkins, tobacco, soybeans and treatment to manure.
U.S. Maximum Allowable Residue Levels for the residues
of Trichlorfon [40 CFR 180.198]: in or on the following
raw agricultural commodities: cattle, fat 0.1ppm (negligible residue); cattle, meat byproducts 0.1ppm (negligible
residue); and cattle, meat 0.1ppm (negligible residue) | | 応用例(製薬) | An organophosphorus compound. It is soluble in water and
stable at room temperature. At higher temperatures it decomposes
to the insecticide dichlorvos. | | 製品名 | AEROL 1 (PESTICIDE)®;
AGROFOROTOX®; ANTHON®; BAY 15922®;
BAYER 15922®; BAYER L 13/59®; BILARCIL®;
BOVINOX®[C]; BRITON®; BRITTEN®; CEKUFON®;
CHLORAK®; CHLOROFTALM®; CICLO-SOM®;
COMBOT®; COMBOT EQUINE®; DANEX®[C];
DEP®; DEPTHON®; DIMETOX®; DIPTEREX®;
DIPTEREX® 50; DIPTEVU®; DITRIFON®; DYLOX®;
DYLOX-METASYSTOX-R®; DYREX®; DYVON®;
EQUINO-ACID®; EQUINO-AID®; FLIBOL E®;
FLIEGENTELLE®; FOROTOX®; FOSCHLOR®;
FOSCHLOR R®; FOSCHLOR R-50®; LEIVASOM®;
LOISOL®; MASOTEN®[C]; MAZOTEN®;
NEGUVON®; NEGUVON A®; PHOSCHLOR R50®;
PROXOL®; RICIFON®; RITSIFON®; SATOX 20WSC®;
SOLDEP®; SOTIPOX®; TRICHLORPHON FN®;
TRINEX®; TUGON®; TUGON FLY BAIT®; TUGON
STABLE SPRAY®; VERMICIDE BAYER 2349®;
VOLFARTOL®; VOTEXIT®; WEC 50®; WOTEXIT® | | 作用機序 | In vivo, metrifonate rapidly rearranges to
O,O-dimethyl O-(2,2-dichlorovinyl) phosphate,
which is a potent inhibitor of schistosome acetylcholinesterase.
This paralyzes the parasites because
of the accumulation of acetylcholine,
which functions as inhibitory transmitter .
Trade name: Bilarcil (Bayer). | | 薬物動態学 | Metrifonate is rapidly absorbed after oral administration,
achieving a peak concentration in plasma within 1–2 h. It
undergoes chemical transformation to dichlorvos, which is
the active molecule. Dichlorvos is rapidly and extensively
metabolized and excreted mainly in the urine. | | 臨床応用 | Urinary schistosomiasis (especially mass chemotherapy control programs) | | 副作用 | Various side effects such as abdominal pain, gastrointestinal
upsets and vertigo occur in many patients. As the worms release their hold of the veins in the bladder they pass through
the blood system to the lungs, where they disintegrate; this
may cause some of the side effects. Cholinesterase levels in
the blood and on erythrocytes are depressed, but the significance
of this is unknown. | | 安全性プロファイル | Poison by ingestion,
inhalation, inti-aperitoneal, subcutaneous,
intravenous, and intramuscular routes.
Moderately toxic by skin contact. Human
systemic effects: true cholinesterase.
Experimental teratogenic and reproductive
effects. Questionable carcinogen with
experimental carcinogenic and tumorigenic
data. Human mutation data reported. An eye
irritant. When heated to decomposition it
emits very toxic fumes of Cland POx. | | 職業ばく露 | Trichlorfon is used as an agricultural and forest insecticide. | | 発がん性 | When rats were fed diets that
contained 0, 50, 100, 200, 250, 400, 500, or 1000 ppm
(equivalent to about 0.5, 12.5, 25, or 50 mg/kg/day) for 17
or 24 months, no treatment-related effects occurred in those
fed 50–250 ppm . Histopathological results suggested
the occurrence of mammary tumors in rats fed 400, 500, and
1000 ppm. In another study, when rats were fed diets containing
0, 50, 250, 500, or 1000 ppm (equivalent to about 2.5,
12.5, 25, or 50 mg/kg/day) trichlorfon for 24 months, no
treatment-related effects other than whole-blood cholinesterase
depression at 1000 ppm occurred . There was no
increase in the incidence of either benign or malignant
tumors, including mammary tumors. | | 環境運命予測 | Soil. Trichlorfon degraded in soil to dichlorvos (alkaline conditions) and desmethyl
dichlorvos (Mattson et al., 1955).
Plant. In cotton leaves, the metabolites identified included dichlorvos, phosphoric acid,
O-demethyl dichlorvos, O-demethyl trichlorfon, methyl phosphate and dimethyl phosphate
(Bull and Ridgway, 1969). Chloral hydrate and trichloroethanol were r
Pieper and Richmond (1976) studied the persistence of trichlorfon in various foliage
following an application rate of 1.13 kg/ha. Concentrations of the insecticide found at day
0 and 14 were 81.7 ppm and 7 ppb for willow foliage, 12.6 ppm and 670 ppb for
Chemical/Physical. At 100°C, trichlorfon decomposes to chloral. Decomposed by hot
water at pH <5 forming dichlorvos (Worthing and Hance, 1991). | | 代謝経路 | The metabolism of trichlorfon has been reviewed by Zayed et al. (1967),
Sawicki (1973) and Zayed (1974). Trichlorfon is a non-systemic insecticide
with favourable mammalian toxicity. There is considerable evidence
that trichlorfon requires in vivu activation via dehydrochlorinatation to
yield dichlorvos which is the active acetylcholinesterase inhibitor. This
reaction is quite facile in slightly basic solution and the subsequent
routes for the metabolism of trichlorfon are apparently the same as those
of dichlorvos. However, there has been considerable controversy on the
role played by this reaction in vivu since many workers have failed to
identify dichlorvos as a metabolite in plants, mammals or insects treated
with trichlorfon. It was realised that trichlorfon was a very much poorer
inhibitor of acetylcholinesterase than dichlorvos and most considered
that its insecticidal activity must be due to metabolism to dichlorvos as
an activating step in an analogous way that phosphorothioates are
metabolised to phosphates. Metcalf et al. (1959) and Miyamoto (1959)
proposed that trichlorfon was totally inactive as an inhibitor of acetylcholinesterase
and that any inhibition seen in vitru was due to some
conversion to dichlorvos during the course of the assay for anticholinesterase
activity. Metcalf et al. (1959) also reported the identification
of dichlorvos in trichlorfon-treated houseflies. This conclusion was
by no means universal and Arthur and Casida (1957) argued that trichlorfon
was the active acetylcholinesterase inhibitor and the identification
of a glucuronide conjugate of trichloroethanol was evidence that the
primary route of stage I metabolism was hydrolysis of the P< bond.
Other work, however, has provided evidence for the in vivu production of
dichlorvos and it seems probable that the failure of some experiments to
detect it is due to its rapid metabolism, resulting in a very low steadystate
concentration. The metabolism of trichlorfon can be envisaged
as being either through a deactivation route via demethylation and/or
conjugation followed by breakdown of the demethylated products
or an activation reaction to yield dichlorvos which is then degraded
via a hydrolytic mechanism to yield dimethyl phosphate and dichloroacetaldehyde
or demethylated by glutathione-S-methyl transferase.
These competing reactions have been investigated in mammals and
insects and it is the balance of activation and degradative metabolism
which confers the favourable mammalian toxicity of trichlorfon in
comparison with that of dichlorvos. | | 代謝 | Trichlorfon administered to mammals is rapidly metabolized and excreted almost completely in the
urine within 6 h. Majormetabolites are dimethyl hydrogen
phosphate, methyl dihydrogen phosphate, and conjugates
of dichloroacetic acid and trichloroethanol. Trichlorfon is
rapidly broken down in soil. | | 輸送方法 | UN2783 Organophosphorus pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. | | Toxicity evaluation | The acute oral LD50 for rats is about 450 mg/kg.
Inhalation LC50 (1 h) for rats is >0.5 mg/L air. NOEL
(2 yr) for rats is 100 mg/kg diet (5 mg/kg/d). ADI is
0.01 mg/kg b.w. | | Degradation | Trichlorfon is subject to hydrolysis and dehydrochlorination. Decomposition
proceeds more rapidly with heating and above pH 6. It is rapidly
converted by alkalis to dichlorvos (2) which is then hydrolysed. DT50 s at pH 4,7 and 9 were 510 days, 46 hours and <30 min respectively at 20 °C.
Photolysis is slow (PM).
Trichlorfon undergoes a facile rearrangement in the presence of mild
base or heat to yield dichlorvos (2) and one mole of HCI (Barthel et al.,
1955; Lorenz et al., 1955; Mattson et al., 1955). This reaction was shown to
be first order in both trichlorfon concentration and [OH-] with a calculated
t1/2 of 5 hours at pH 7.0 (37 °C) (Miyamoto, 1959). A mechanism for
this reaction is shown in Scheme 1. | | 不和合性 | This chemical may be characterized as an organo-phosphate or-chlorine compound. Organophosphates are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducin g agents such as hydrideds and active metals. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.Alkaline materials: lime, lime sulfur, etc. Corrosive to iron, steel and possibly to other metals. | | 廃棄物の処理 | Add a combustible solvent and burn in a furnace equipped with an afterburner and an alkali scrubber.In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. |
|