|
ChemicalBook Optimization Suppliers |
| 名前: |
Infinity SCI Gold |
| 電話番号: |
400-400-1062016 17800804092 |
| 電子メール: |
admin@infsci.com |
|
| 化学名: | リチウムトリエチルボロヒドリド | | 英語化学名: | Lithium Triethylborohydride | | 别名: | lithiumtriethylborohydride,calselect?lt,;lithiumtriethylborohydride,calselect?lt,intetrahydrofuran;Lithium triethylborohydrideLithium triethylhydroborate;Lithium triethylborahydride (super-hydride);Super-Hydride solution,Lithium triethylborohydride solution;Lithium triethylborohydride, 1M solution in THF, AcroSeal;LithiuM triethylborohydride, 1M solution in THF, AcroSeal 100ML;LithiuM triethylborohydride, 1M solution in THF, AcroSeal 800ML | | CAS番号: | 22560-16-3 | | 分子式: | C6H13BLi | | 分子量: | 102.92 | | EINECS: | 245-076-8 | | カテゴリ情報: | | | Mol File: | 22560-16-3.mol | 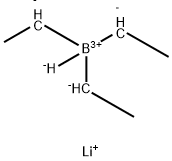 |
| 融点 | 66-67° (Binger); mp 78-83° (dec) (Brown, 1977) | | 比重(密度) | 0.892 g/mL at 25 °C | | 闪点 | 1 °F | | 貯蔵温度 | Flammables + water-Freezer (-20°C)e area | | 溶解性 | Miscible with tetrahydrofuran and benzene. | | 外見 | Liquid | | 色 | Colorless to light gray | | Sensitive | Air & Moisture Sensitive | | Merck | 14,5544 | | BRN | 4151240 | | 暴露限界値 | ACGIH: TWA 50 ppm; STEL 100 ppm (Skin)
OSHA: TWA 200 ppm(590 mg/m3)
NIOSH: IDLH 2000 ppm; TWA 200 ppm(590 mg/m3); STEL 250 ppm(735 mg/m3) | | CAS データベース | 22560-16-3(CAS DataBase Reference) |
| | リチウムトリエチルボロヒドリド Usage And Synthesis |
| 用途 | 水素化トリエチルホウ素リチウム(すいそかトリエチルホウそリチウム、lithium triethylborohydride, LiTEBH)とは有機金属化合物の一種で、ヒドリド還元に用いられる試薬。求核性や選択性が極めて強いヒドリド化剤。 | | 説明 | Lithium triethylborohydride is the organoboron compound with the formula LiEt3BH. Commonly referred to as LiTEBH or Superhydride, it is a powerful reducing agent used in organometallic and organic chemistry. It is a colorless or white liquid but is typically marketed and used as a THF solution. The related reducing agent sodium triethylborohydride is commercially available as toluene solutions.
LiBHEt3 is a stronger reducing agent than lithium borohydride and lithium aluminium hydride. | | 化学的特性 | colorless to light grey cloudy solution | | 使用 | LiTEBH can be used as a reagent:
- To reduce alkyl halides to alkanes via dehydrogenation reactions.
- For the selective reduction of epoxides to Markovnikov alcohols.
- To reduce tosylates or mesylates primary alcohols to hydrocarbons.
- For reductive cyclization reactions for the preparation of useful intermediates.
- In the synthesis of hepta(manno-3-deoxy-6-O-t-butyldimethylsilyl)-β-cyclodextrin by reduction of hepta(manno-2,3-anhydro-6-O-t-butyldimethylsilyl)-β-cyclodextrin.
- For hydrodefluorination of C-F bonds using Ni catalyst.
- To prepare alkynyl alcohols from cleavage of cyclic keto-vinyl triflates.
- In the stereoselective reduction of bicyclic imides, isoquinolines, and pyridines.
- To prepare tungsten and molybdenum hydride complexes.
| | 使用 | Powerful and selective reducing agent.
As a reducing agentLithium triethylborohydride is used as a powerful reducing agent in organic synthesis for the conversion of carbonyl compounds to alcohols. It is also used in the preparation of alkynyl alcohols from the cleavage of cyclic keto-vinyl triflates. It is also used in the reduction of esters and lactones to alcohol and diol respectively. Further, it is used to prepare 1-methylcyclohexanol and 1,4-butanediol from 1,2-epoxybutane and gamma-butyrolactone respectively. It is also utilized for reductive cleavage of mesylates and tosylates in synthetic organic chemistry. | | 製造方法 | LiBHEt3 is prepared by the reaction of lithium hydride (LiH) and triethylborane (Et3B) in tetrahydrofuran (THF):
LiH + Et3B → LiEt3BH
Its THF solutions are stable indefinitely in the absence of moisture and air. | | 主な応用 | Lithium Triethylborohydride (LiTEBH) is widely used as a powerful and selective reducing agent that shows super hydride activity in organic synthesis.
LiTEBH can be used as a reagent:
To reduce alkyl halides to alkanes via dehydrogenation reactions.
For the selective reduction of epoxides to Markovnikov alcohols.
To reduce tosylates or mesylates primary alcohols to hydrocarbons.
For reductive cyclization reactions for the preparation of useful intermediates.
In the synthesis of hepta(manno-3-deoxy-6-O-t-butyldimethylsilyl)-β-cyclodextrin by reduction of hepta(manno-2,3-anhydro-6-O-t-butyldimethylsilyl)-β-cyclodextrin.
For hydrodefluorination of C-F bonds using Ni catalyst.
To prepare alkynyl alcohols from cleavage of cyclic keto-vinyl triflates.
In the stereoselective reduction of bicyclic imides, isoquinolines, and pyridines.
To prepare tungsten and molybdenum hydride complexes. | | 一般的な説明 | Super-Hydride? solution (Lithium triethylborohydride or LiTEBH) is widely used as a powerful and selective reducing agent that shows super hydride activity in organic synthesis. | | 予防処置 | Moisture sensitive. Air sensitive. Incompatible with water and strong oxidizing agents. | | 参考文献 | Tamang, S.; Kim, K.; Choi, H.; Kim, Y.; Jeong, S. Synthesis of colloidal InSb nanocrystals via in situ activation of InCl3. Dalton Trans. 2015, 44 (38), 16923-16928.
Kandapallil, B.; Colborn, R. E.; Bonitatibus, P. J.; Johnson, F. Synthesis of high magnetization Fe and FeCo nanoparticles by high temperature chemical reduction. J. Magn. Magn. Mater. 2015, 378, 535-538. |
|