|
ChemicalBook Optimization Suppliers |
|
| 化学名: | 酢酸メドロキシプロゲステロン | | 英語化学名: | Medroxyprogesterone Acetate | | 别名: | MEDROXYPROGESTERONE ACETATE, FOR PERFORMANCE TEST EP STANDARD;Medroxyprogesterone Acetate, Micronized;17α-Acetoxy-6α-methylprogesterone, 6α-Methyl-17α-acetoxyprogesterone, 6α-Methyl-17α-hydroxyprogesterone acetate, Depo-provera, 17α-Hydroxy-6α-methyl-4-pregnene-3,20-dione 17-acetate;(6-alpha)-Pregn-4-ene-3,20-dione, 17-(acetyloxy)-6-methyl;Pregn-4-ene-3,20-dione, 17-(acetyloxy)-6-methyl-, (6.alpha.)-;17α-acetoxy-6α-methylprogesterone;6a-methyl-17a-acetoxyprogesterone;MEDROXYPROGESTERONEACETATE,MICRONIZED,USP | | CAS番号: | 71-58-9 | | 分子式: | C24H34O4 | | 分子量: | 386.52 | | EINECS: | 200-757-9 | | カテゴリ情報: | Antitumors for Research and Experimental Use;Biochemistry;Hydroxyketosteroids;Steroids;PROVERA;API;Hormone Drugs;Intermediates & Fine Chemicals;Pharmaceuticals;Steroid and Hormone;71-58-9 | | Mol File: | 71-58-9.mol | 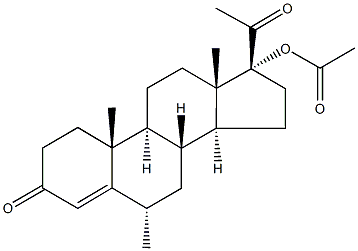 |
| 融点 | 206-207 °C(lit.) | | 比旋光度 | D +61° (in chloroform) | | 沸点 | 432.7°C (rough estimate) | | 比重(密度) | 1.0346 (rough estimate) | | 屈折率 | 48 ° (C=1, Dioxane) | | 貯蔵温度 | Sealed in dry,2-8°C | | 溶解性 | Practically insoluble in water, freely soluble in methylene chloride, soluble in acetone, sparingly soluble in ethanol (96 per cent) | | 色 | White | | 水溶解度 | <0.1 g/100 mL at 23 ºC | | Merck | 13,5817 | | BRN | 2066112 | | BCS Class | 2,3,1 | | 安定性: | Stable, but weakly air and light sensitive. Incompatible with strong oxidizing agents. | | CAS データベース | 71-58-9(CAS DataBase Reference) | | IARC | 2B (Vol. 21, Sup 7) 1987 | | EPAの化学物質情報 | Medroxyprogesterone acetate (71-58-9) |
| | 酢酸メドロキシプロゲステロン Usage And Synthesis |
| 外観 | 白色~わずかにうすい褐色, 結晶~粉末 | | 溶解性 | クロロホルム,アセトンに可溶、エーテル,水に不溶。1,4-ジオキサンに溶ける。 | | 用途 | 薬理研究用。 | | 用途 | 黄体ホルモン剤です。 | | 用途 | 黄体ホルモン物質です。エス
トロゲン抑制作用を示します。 | | 効能 | 抗悪性腫瘍薬, 避妊薬, プロゲステロン受容体作動薬 | | 商品名 | ヒスロン (協和発酵キリン); ヒスロン (協和発酵キリン); プロベラ (ファイザー) | | 説明 | Medroxyprogesterone Acetate, also known as Medroxyprogesterone 17-acetate or MPA, is a synthetic progestogen and a steroidal progestin. It is derived from the human hormone progesterone. It prevents fertilization and increases the rate of transport of eggs from the fallopian tubes to the uterus in female ferrets when administered prior to ovulation. Medroxyprogesterone 17-acetate reversibly blocks ovulation in rats when injected on the last day of diestrus. It also has anti-androgenic activity in rats, decreasing plasma testosterone levels via induction of hepatic testosterone reductase activity. Medroxyprogesterone 17-acetate exhibits immunosuppressive effects in vitro and in vivo, inhibiting the production of IFN-γ by CD2/CD3/CD28-stimulated peripheral blood mononuclear cells (PBMCs) at concentrations ≥10 nM and extending the survival of rabbit skin allografts. Injectable formulations containing medroxyprogesterone 17-acetate have been used as contraceptives. | | 化学的特性 | White or almost white, crystalline powder. | | Originator | Provera,Upjohn,US,1959 | | 使用 | Medroxyprogesterone Acetate is a synthetic progesterone receptor agonist that is used to treat amenorrhea (unusual stopping of menstrual periods) and abnormal uterine bleeding. | | 定義 | ChEBI: Medroxyprogesterone acetate is an acetate ester resulting from the formal condensation of the 17alpha-hydroxy group of medroxyprogesterone with the carboxy group of acetic acid. A widely used progestin in menopausal hormone therapy and in progestogen-only birth control. It has a role as a progestin, an androgen, a female contraceptive drug, a synthetic oral contraceptive, an adjuvant, an inhibitor, an antioxidant and an antineoplastic agent. It is a steroid ester, an acetate ester, a 20-oxo steroid, a 3-oxo-Delta(4) steroid and a corticosteroid. It is functionally related to a medroxyprogesterone. | | brand name | Amen (Amarin);
Curretab (Solvay Pharmaceuticals); Cycrin (ESI); Provera
(Pharmacia & Upjohn);Clinovie;Cliovir;Dep0-clinover;Dep0-map;Depcorlutin;Depo-prodasone;Depo-progevera;Depo-promone;Deporone;Dugen;Farlurin;Farlutale;Gesinal;Gestapuran;Gestapuron;G-farlutal;Hysron;Intex;Luteocrin orale;Luteodione;Luteos;Lutoporal;Metigestene;Nadigest;Nogest;Onco-provera;Perlutest;Petogen;Piermap;Povera;Promone-e;Pronone;Proverone;Provest;Sindomens;Sodelut "g";Supprestal;Verafen;Veramix plus v. | | Therapeutic Function | Progestin | | 世界保健機関(WHO) | A depot preparation containing 150 mg medroxyprogesterone
acetate was introduced over 20 years ago for use as a long-acting injectable
contraceptive. Subsequently, positive results of carcinogenicity studies carried out
in beagle bitches led to refusal of registration in the United States. These findings
were later considered irrelevant to contraceptive use in women and the drug was
approved by the Food and Drug Administration. Menstrual irregularities are the
most common adverse effect associated with depot medroxyprogesterone acetate.
Risk-benefit judgements differ significantly from country to country, having regard
to differing national circumstances. The preparation is, however, widely available
and is included in the WHO Model List of Essential Drugs.
(Reference: (WHTAC4) The Use of Essential Drugs, 4th Report of the WHO Expert
Committee, 796, , 1990) | | 一般的な説明 | Medroxyprogesterone acetate is an odorless white to off-white microcrystalline powder. It is a synthetic, acetate derivative of the sex hormone progesterone. (NTP, 1992) | | 空気と水の反応 | Medroxyprogesterone 17-acetate is sensitive to prolonged exposure to air and light. Insoluble in water. | | 反応プロフィール | Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides. | | 危険性 | Possible carcinogen. | | 火災危険 | Flash point data for Medroxyprogesterone 17-acetate are not available; however, Medroxyprogesterone 17-acetate is probably combustible. | | Biochem/physiol Actions | Medroxyprogesterone 17-acetate (MPA) is a synthetic progestin used as a contraceptive, in hormone replacement therapy and for the treatment of endometriosis. It is a more potent progestin that the nonacetylated form. | | 臨床応用 | Progestogen:
Cachexia (unlicensed), contraception, epilepsy, male
hypersexuality, malignant neoplasms, respiratory
disorders, sickle-cell disease, dysfunctional uterine
bleeding, endometriosis | | 安全性プロファイル | Suspected carcinogen
with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data.
Human systemic effects by intravenous
route: increased intraocular pressure. Human teratogenic effects by an unspecified
route: developmental abnormalities of the
urogenital system. Human reproductive
effects by multiple routes:
spermatogenesis, menstrual cycle changes or
dlsorders, postpartum effects, female fertility
effects, abortion, newborn behavioral
effects. Human mutation data reported.
Experimental reproductive effects. A drug
for the treatment of secondary amenorrhoea
and dysfunctional uterine bleeding. When
heated to decomposition it emits acrid
smoke and irritating fumes. | | Veterinary Drugs and Treatments | In cats, MPA has been used when either castration is ineffective or
undesirable to treat sexually dimorphic behavior problems such as
roaming, inter-male aggressive behaviors, spraying, mounting, etc.
MPA has also been used as a tranquilizing agent to treat syndromes
such as feline psychogenic dermatitis and alopecia, but treatment
with “true” tranquilizing agents may be preferable.
In humans, parenteral MPA has been used as a long-acting
contraceptive in females, to decrease sexually deviant behavior in
males, and as an antineoplastic agent for some carcinomas (see
Pharmacology section above). Oral MPA is used in human females
to treat secondary amenorrhea and to treat abnormal uterine bleeding
secondary to hormone imbalances. | | 代謝 | Among the first of these substituted 17α-acetoxyprogesterone analogues to be utilized therapeutically was medroxyprogesterone
acetate, a 6α-methyl progesterone analogue. This analogue is 25-fold more active than ethisterone. Following oral
administration, medroxyprogesterone acetate is completely and rapidly deacetylated by first-pass metabolism to
medroxyprogesterone. Medroxyprogesterone is extensively metabolized via pathways similar to those for progesterone, except for
6α-hydroxylation. Most medroxyprogesterone acetate metabolites are excreted in the urine, primarily as glucuronide conjugates.
Plasma protein binding for medroxyprogesterone is approximately 86%, primarily to serum albumin, with no binding to SHBG. | | 参考文献 | 1. Chang, M.C. Effects of medroxyprogesterone acetate and of ethinyl oestradiol on the fertilization and transportation of ferret eggs. J. Reprod. Fertil. 13(1), 173-174 (1967). DOI:10.1530/JRF.0.0130173
2. Dickmann, Z. Short-and long-term effects of a single injection of depo-medroxyprogesterone acetate (provera) on the vaginal smear, ovulation and mating in the rat. J. Reprod. Fertil. 32(3), 447-451 (1973). DOI:10.1530/JRF.0.0320447
3. Albin, J., Vittek, J., Gordon, G.G., et al. On the mechanism of the anti-androgenic effect of medroxyprogesterone acetate. Endocrinology 93(2), (1973). DOI:10.1210/ENDO-93-2-417
4. Huijbregts, R.P., Michel, K.G., and Hel, Z. Effect of progestins on immunity: Medroxyprogesterone but not norethisterone or levonorgestrel suppresses the function of T cells and pDCs. Contraception 90(2), 123-129 (2014). DOI:10.1016/j.contraception.2014.02.006
5. Turcotte, J.G., Haines, R.F., Brody, G.L., et al. Immunosuppression with medroxyprogesterone acetate. Transplantation 6(2), 248-260 (1968). DOI:10.1097/00007890-196803000-00010
6. Hofmeyr, G.J., Singata-Madliki, M., Lawrie, T.A., et al. Effects of the copper intrauterine device versus injectable progestin contraception on pregnancy rates and method discontinuation among women attending termination of pregnancy services in South Africa: A pragmatic randomized controlled trial. Reprod. Health 13, 42 (2016). DOI:10.1186/s12978-016-0153-9
7. Thomas CP, Liu KZ, Vats HS. Medroxyprogesterone acetate binds the glucocorticoid receptor to stimulate alpha-ENaC and sgk1 expression in renal collecting duct epithelia. Am J Physiol Renal Physiol. 2006 Feb;290(2):F306-12. Epub 2005 Sep 27. DOI:10.1152/AJPRENAL.00062.2005
8. Braden BB, Talboom JS, Crain ID, Simard AR, Lukas RJ, Prokai L, Scheldrup MR, Bowman BL, Bimonte-Nelson HA. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem. 2010Mar;93(3):444-53. DOI:10.1016/j.nlm.2010.01.002 |
|