|
ChemicalBook Optimization Suppliers |
|
| 化学名: | 硫酸鉄(II)/水和物,(1:x) | | 英語化学名: | Ferrous sulfate monohydrate | | 别名: | Ferrous Sulphate Monohydrate(Powder / Granular);Sulfuric acid iron(II)/hydrate,(1:x) salt;Ferrous sulfate hydrate, unspecified (FeSO4.xH2O);Dried Ferrous Sulfate, Powder, USP;Iron(Ⅱ) sulfate hydrate;Sulfuric acid, iron(II) salt (1:1), hydrate;Iron(II) sulfate hydrate puriss., meets analytical specification of Ph. Eur., BP, exsiccated, 86.0-89.0% FeSO4 basis;IRON (II) SULPHATE HYDRATED | | CAS番号: | 13463-43-9 | | 分子式: | FeH2O5S | | 分子量: | 169.92 | | EINECS: | 603-840-1 | | カテゴリ情報: | Industry grade;Catalysis and Inorganic Chemistry;Chemical Synthesis;Iron Salts;Metal and Ceramic Science;Salts | | Mol File: | 13463-43-9.mol | 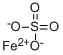 |
| 融点 | 64 °C | | 比重(密度) | 1.9 g/mL at 25 °C(lit.) | | 蒸気圧 | 14.6 mm Hg ( 25 °C) | | 貯蔵温度 | 2-8°C | | 溶解性 | Slowly but freely soluble in water, very soluble in boiling water, practically insoluble in ethanol (96 per cent). | | 外見 | solid | | PH | 3.0-4.0 (20℃, 5%) | | Merck | 13,4091 | | Dielectric constant | 32.4(Ambient) | | CAS データベース | 13463-43-9(CAS DataBase Reference) |
| | 硫酸鉄(II)/水和物,(1:x) Usage And Synthesis |
| 効能 | 貧血治療薬, 造血薬, 鉄補充薬 | | 商品名 | フェロ・グラデュメット (マイランEPD) | | 化学的特性 | Iron(II) sulfate hydrate, also known as ferrous sulfate hydrate, is Off-white to beige powder. Astringent odor. Mainly composed of FeSO4.H2O and contains a small amount of FeSO4.4H2O. harder to oxidize, and easier to store than crystalline ferrous sulfate. The aqueous solution is acidic and turbid, gradually producing a yellow-brown precipitate. It absorbs water into heptahydrate in humid air. Heating to 120 ℃ does not lose water. Boiling with water can be converted into alkali sulfate. Slowly soluble in cold water (26.6g/100ml, 20℃), rapidly dissolved when heated. Insoluble in ethanol. Almost insoluble in 50% sulfuric acid. | | 使用 | Iron(II) sulfate hydrate has been used to prepare ferrous ion solution to study the influence of iron ions on the thermal decomposition behaviour of hydroxylamine/water solutions. | | 定義 | ChEBI: Iron(2+) sulfate monohydrate is a hydrate that is the monohydrate form of iron(2+) sulfate. It is obtained from the heptahydrate by heating above 65℃. It is used as a source of iron in the treatment of iron-deficiency anaemia (generally in solid-dosage treatments; for liquid-dosage treatments, the heptahydrate is normally used). It has a role as a nutraceutical, an anti-anaemic agent and a reducing agent. It is a hydrate and an iron molecular entity. It contains an iron(2+) sulfate (anhydrous). | | 製造方法 | It is obtained by drying crystallized ferrous sulfate into powder at 40℃. Heating to 45 ~ 50 ℃ when dissolved in the water of crystallization and liquefaction, while stirring and slowly evaporating the water of crystallization. The limit of dry weight loss is 35%~36%, generating a small particle state fine powder, made of powder, sealed and stored. | | 农业用途 | Ferrous sulphate heptahydrate (FeSO4.7H2O), also known as green vitriol or copperas, contains 19% iron and is a common iron fertilizer. It is the best known ferrous salt. |
|