|
ChemicalBook Optimization Suppliers |
| 名前: |
BEST-REAGENT |
| 電話番号: |
400-1166-196 18981987031 |
| 電子メール: |
cdhxsj@163.com |
|
| 融点 | 54-56 °C | | 沸点 | 300 °C | | 比重(密度) | 0.92 g/mL at 25 °C (lit.) | | 屈折率 | n20/D 1.51 | | 外見 | slab/chunk | | 安定性: | Stability Combustible. Incompatible with strong oxidizing agents. | | InChI | InChI=1S/C4H8/c1-4(2)3/h1H2,2-3H3 | | InChIKey | VQTUBCCKSQIDNK-UHFFFAOYSA-N | | SMILES | C=C(C)C | | EPAの化学物質情報 | Polyisobutylene (9003-27-4) |
| | ポリ(イソブチルン) Usage And Synthesis |
| 定義 | 本品は、イソブチレンの重合体であり、次の化学式で表される。 | | 解説 | ポリイソブチレン,一般式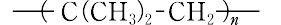 で表されるイソブテンの重合体.高温で重合したものは,重合度が低い粘ちゅう液体である.-100 ℃ に近い極度の低温下で,塩化アルミニウムや三フッ化ホウ素などのカチオン触媒で重合すると数十万の分子量になり,ゴム状弾性([別用語参照]ゴム弾性)を示す.高重合体の用途は主として合成ゴムであり,分子中に不飽和結合をもたないため化学的に不活性で,オゾン,酸,アルカリなどに対する抵抗性が強い.また,ガスの透過性が小さいのが特徴である.一般に,加硫を容易にするため,少量のジエン類(主としてイソプレン)を共重合して用いる.高重合体はタイヤ用チューブとして,低重合体は防湿シールなどに使用されている. で表されるイソブテンの重合体.高温で重合したものは,重合度が低い粘ちゅう液体である.-100 ℃ に近い極度の低温下で,塩化アルミニウムや三フッ化ホウ素などのカチオン触媒で重合すると数十万の分子量になり,ゴム状弾性([別用語参照]ゴム弾性)を示す.高重合体の用途は主として合成ゴムであり,分子中に不飽和結合をもたないため化学的に不活性で,オゾン,酸,アルカリなどに対する抵抗性が強い.また,ガスの透過性が小さいのが特徴である.一般に,加硫を容易にするため,少量のジエン類(主としてイソプレン)を共重合して用いる.高重合体はタイヤ用チューブとして,低重合体は防湿シールなどに使用されている. | | 化粧品の成分用途 | 非水系増粘剤、結合剤、皮膜形成剤 | | 主な用途/役割 | イソブチレンを重合させたもの。木材用接着剤、粘着剤原料として使用される。 | | 分類 | 高分子量ポリイソブチレン 重合度1000以上。ゴム状固体であるが、重合体中に不飽和結合をもたないので化学的に安定。ゴム配合剤に用いられる。また、少量(約3%)のイソプレンと共重合させたものはブチルゴムとよばれる。 中分子量ポリイソブチレン、低分子量イソブチレン 中分子量ポリイソブチレンは重合度は数百程度。粘い液体ないし半固体で、接着剤、シーラント、ワックス、ポリエチレンの改質剤などに用いられるほか、天然ゴムや合成ゴムに配合して耐老化性・耐オゾン性などの改善に用いるなど広い用途がある。低分子量ポリイソブチレンは重合度10程度。分子量500~1300程度の粘い液体。潤滑油または潤滑油の粘度調節剤として用いられる。 | | 化学的特性 | Polyisobutylene is composed of long-chain hydrocarbon formed by polymerization of isobutene, and is extremely stable under normal conditions. It is transparent non-noxious high-consistency semi-solid polymer free of impurities. | | 化学的特性 | The physical properties of polyisobutene are very dependent on molecular
weight. Polymers with average molecular weight (Mw) of about 15 000 are sticky viscous liquids whilst those with molecular weight of 100000-200000
are rubber-like, resembling unmilled crepe rubber.
Polyisobutene is non-crystalline when unstretched and is therefore soluble
at room temperature in hydrocarbons and halogenated hydrocarbons. The
material is resistant to most acids, alkalis and aqueous solutions, as would be
expected from its saturated hydrocarbon structure and absence of tertiary
hydrogen atoms. The lack of tertiary hydrogen atoms renders polyisobutene
more resistant to oxidation than polypropylene; also, the less numerous and
partially shielded methylene groups in polyisobutene are less reactive than
those in polyethylene. However, polyisobutene is rather susceptible to thermal degradation since chain scission is favoured by the greater stability of the
resultant tertiary free radical:
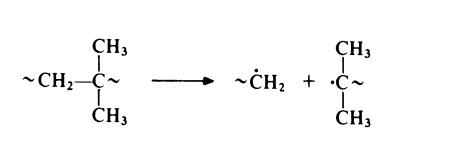 Polyisobutene may be chlorinated but the reaction is accompanied by
severe degradation. Polyisobutene may be chlorinated but the reaction is accompanied by
severe degradation.
A limitation of polyisobutene is its tendency to cold flow and, as a result,
the polymer finds little use in self-supporting form. Applications are restricted
mainly to adhesives, fabric and paper coatings, and blends with other polymers. Low molecular weight polyisobutene is also used in caulking compounds. | | 使用 | polyisobutene (hydrogenated) is an emollient.
Polyisobutylene, sometimes called butyl rubber, and other times PIB, is a vinyl polymer. It's very similar to polyethylene and polypropylene in structure, except that every other carbon is substituted with two methyl groups. It is made from the monomer isobutylene, by cationic vinyl polymerization.
Polyisobutylene is a synthetic rubber, or elastomer. It's special because it's the only rubber that's gas impermeable. That is, it's the only rubber that can hold air for long periods of time. You may have noticed that balloons will go flat after a few days. This is because they are made of polyisoprene, which is not gas impermeable. Because polyisobutylene will hold air, it is used to make things like the inner tubes, liner layers of tires, and the inner liners of basketballs. | | 定義 | ChEBI: A polymer composed of repeating 1,1-dimethylethylene units. | | 製造方法 | Silicone rubbers are prepared as follows:
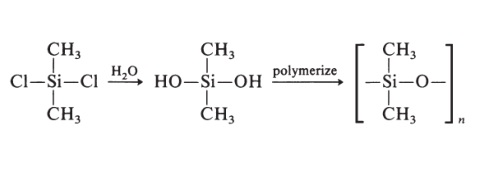
Other groups may replace the methyl groups. Silicone rubbers have excellent ozone and weathering resistance, good electrical properties, and good adhesion to metal. |
|