|
ChemicalBook Optimization Suppliers |
|
| 化学名: | セルメチニブ | | 英語化学名: | Selumetinib | | 别名: | ARRY 142886;AZD 6244;Selumetinib
AZD624;5-[(4-Bromo-2-chlorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-car;Selumetinib;5-(4-Bromo-2-chlorophenylamino)-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzo[d]imidazole-6-carboxamide;6-(4-Bromo-2-chlorophenylamino)-7-fluoro-N-(2-hydroxyethoxy)-3-methyl-3H-benzo[d]imidazole-5-carboxamide;SeluMatinib(5-[(4-BroMo-2-chlorophenyl)aMino]-4-fluoro-N-(2-hydroxyethoxy)-1-Methyl-1H-benziMidazole-6-carboxaMide | | CAS番号: | 606143-52-6 | | 分子式: | C17H15BrClFN4O3 | | 分子量: | 457.68 | | EINECS: | 207-313-3 | | カテゴリ情報: | MAPK;Inhibitors;Antineoplastic;apis;Aromatics;Heterocycles;Inhibitor;Intermediates & Fine Chemicals;Pharmaceuticals;API;Research | | Mol File: | 606143-52-6.mol | 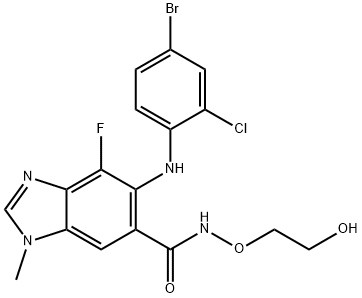 |
| 融点 | >219°C (dec.) | | 比重(密度) | 1.69 | | 貯蔵温度 | -20° | | 溶解性 | Soluble in DMSO (up to 50 mg/ml) or in Ethanol (up to 2 mg/ml) | | 外見 | Beige powder. | | 酸解離定数(Pka) | 14.20±0.10(Predicted) | | 色 | White | | 安定性: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 2 months. |
| | セルメチニブ Usage And Synthesis |
| 効能 | 抗悪性腫瘍薬, マイトジェン活性化細胞外シグナル関連キナーゼ(MEK)阻害薬 | | 説明 | Selumetinib (AZD6244; ARRY-142886) is an oral MEK inhibitor. In a random�ized trial, NSCLC patients with wild-type KRAS were randomized to erlotinib
alone or combination therapy with selumetinib, while mutant KRAS patients were
randomized to selumetinib alone or combination therapy. The primary end points
were PFS for the KRAS wild-type cohort and objective response rate (ORR) for the
KRAS mutant cohort. Results were not impressive, with no PFS difference in the
KRAS wild-type arm (2.4 vs. 2.1?months) and no ORR difference in the KRAS�mutated subgroup (0% vs. 10%). A planned trial of selumetinib in combination
with the anti-PD-L1 antibody durvalumab has since been suspended (NCT03004105). | | 使用 | It is a tight-binding, uncompetitive inhibitor of mitogen-activated protein kinase kinases (MEK) 1 and 2 currently in clinical development. It is useful as biomarker in human lung cancer cell. Potent MEK inhibitor. | | 定義 | ChEBI: A member of the class of benzimidazoles that is 1-methyl-1H-benzimidazole which is substituted at positions 4, 5, and 6 by fluorine, (4-bromo-2-chlorophenyl)amino, and N-(2-hydroxyethoxy)aminocarbonyl groups, respectiv
ly. It is a MEK1 and MEK2 inhibitor. | | target | MEK1 | | 貯蔵 | Store at -20°C | | 参考文献 | 1) Davies?et al. (2007),?AZD6244(ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamics relationship, and potential for combination in preclinical models; Mol. Cancer Ther.,?6?2209
2) Yeh?et al. (2007),?Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor; Clin. Cancer Res.,?13?1576
3) Catalanotti?et al. (2013),?Phase II trial of MEK inhibitor selumetinib(AZD6244) in patients with BRAFV600E/K-mutated melanoma; Clin. Cancer Res.,?19?2257
4) O’Neil?et al. (2011),?Phase II study of the mitogen-activated protein kinase 1/2 inhibitor selumetinib in patients with advanced hepatocellular carcinoma; J. Clin. Oncol.,?29?2350
5) Khurum?et al. (2012),?A phase I dose escalation study of oral MK-2206 (allosteric Akt inhibitor) with oral selumetinib (AZD6244)(MEK 1/2 inhibitor) in patients with advanced or metastatic solid tumors; J. Clin. Oncol.,?30?e13599
6) Hainsworth?et al. (2010),?A phase II, open label, randomized study to assess the efficacy and safety of AZD6244 versus pemetrexed in patients with non-small cell lung cancer who have failed one or two prior chemotherapeutic regimens; J. Thorac. Oncol.,?5?1630
7) Bodoky?et al. (2012),?A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy; Invest. New Drugs,?30?1216 |
|